Label: CK ONE 3-IN-1 FACE MAKEUP WITH SPF 8 SUNSCREEN- titanium dioxide liquid
-
Contains inactivated NDC Code(s)
NDC Code(s): 66184-410-01, 66184-410-02 - Packager: Coty US LLC
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: OTC monograph not final
DISCLAIMER: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
Drug Label Information
Updated December 8, 2011
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
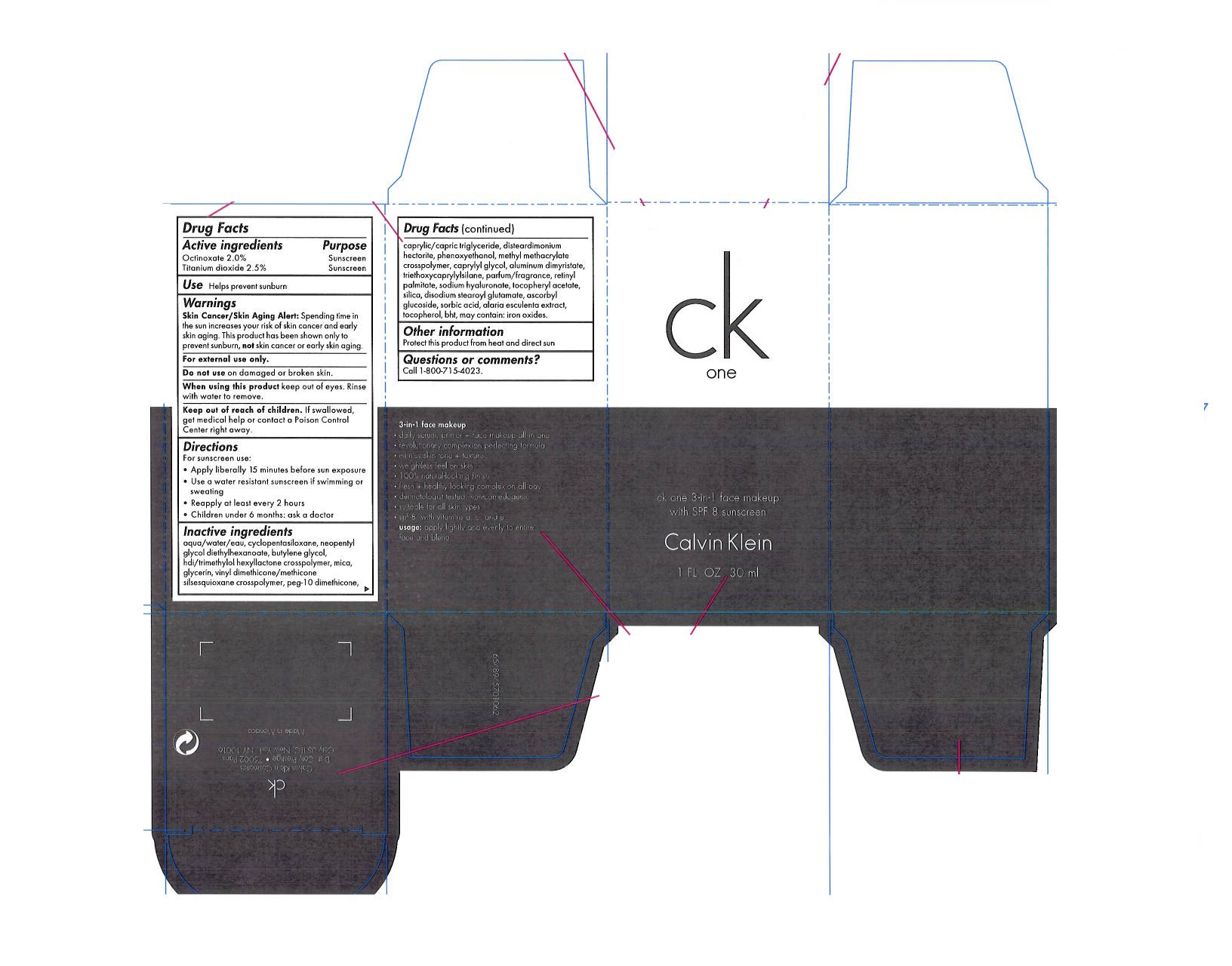
- ACTIVE INGREDIENT
- PURPOSE
- KEEP OUT OF REACH OF CHILDREN
- INDICATIONS & USAGE
- WARNINGS
- DOSAGE & ADMINISTRATION
-
INACTIVE INGREDIENT
water, cyclopentasiloxane, neopentyl, glycol diethylhexanoate, butylene glycol, hdi/trimethylol hexyllactone crosspolymer, mica, glycerin, vinyl dimethicone/methicone silsesquioxane crosspolymer, peg-10 dimethicone, caprylic/capric triglyceride, disteardimonium hectorite, phenoxyethanol, methyl methacrylate crosspolymer, caprylyl glycol, aluminum dimyristate, triethoxycaprylylsilane, parfum/fragrance, retynyl palmitate, sodium hyaluronate, tocopheryl acetate, silica, disodium stearoyl glutamate, ascorbyl glucoside, sorbic acid, alaria esculenta extract, tocopherol, bht, may contain: iron oxides.
- PRINCIPAL DISPLAY PANEL
- PRINCIPAL DISPLAY PANEL
- PRINCIPAL DISPLAY PANEL
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
CK ONE 3-IN-1 FACE MAKEUP WITH SPF 8 SUNSCREEN
titanium dioxide liquidProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:66184-410 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Titanium Dioxide (UNII: 15FIX9V2JP) (Titanium Dioxide - UNII:15FIX9V2JP) Titanium Dioxide 7.5 mL in 30 mL Octinoxate (UNII: 4Y5P7MUD51) (Octinoxate - UNII:4Y5P7MUD51) Octinoxate 0.6 mL in 30 mL Inactive Ingredients Ingredient Name Strength Water (UNII: 059QF0KO0R) Cyclomethicone 5 (UNII: 0THT5PCI0R) Neopentyl Glycol (UNII: QI80HXD6S5) Butylene Glycol (UNII: 3XUS85K0RA) PEG-10 Dimethicone (600 CST) (UNII: 8PR7V1SVM0) Tricaprin (UNII: O1PB8EU98M) Phenoxyethanol (UNII: HIE492ZZ3T) Methyl Methacrylate (UNII: 196OC77688) Caprylyl Glycol (UNII: 00YIU5438U) Aluminum Dimyristate (UNII: J2KA067N9O) Triethoxycaprylylsilane (UNII: LDC331P08E) Vitamin A Palmitate (UNII: 1D1K0N0VVC) Hyaluronate Sodium (UNII: YSE9PPT4TH) Alpha-Tocopherol Acetate (UNII: 9E8X80D2L0) Silicon Dioxide (UNII: ETJ7Z6XBU4) Disodium Stearoyl Glutamate (UNII: 45ASM2L11M) Sorbic Acid (UNII: X045WJ989B) Alaria Esculenta (UNII: EJ9JK8J58D) Tocopherol (UNII: R0ZB2556P8) Butylated Hydroxytoluene (UNII: 1P9D0Z171K) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:66184-410-01 30 mL in 1 CONTAINER 2 NDC:66184-410-02 30 mL in 1 BOX Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph not final part352 02/01/2012 Labeler - Coty US LLC (789573201) Registrant - Coty Inc. (958662223) Establishment Name Address ID/FEI Business Operations Lancaster S.A.M. 401011325 manufacture