Label: LOREAL PARIS INFALLIBLE UP TO 24 H FRESH WEAR FOUNDATION BROAD SPECTRUM SPF 25 SUNSCREEN- octinoxate and titanium dioxide liquid
- NDC Code(s): 49967-009-01
- Packager: L'Oreal USA Products Inc
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated December 29, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
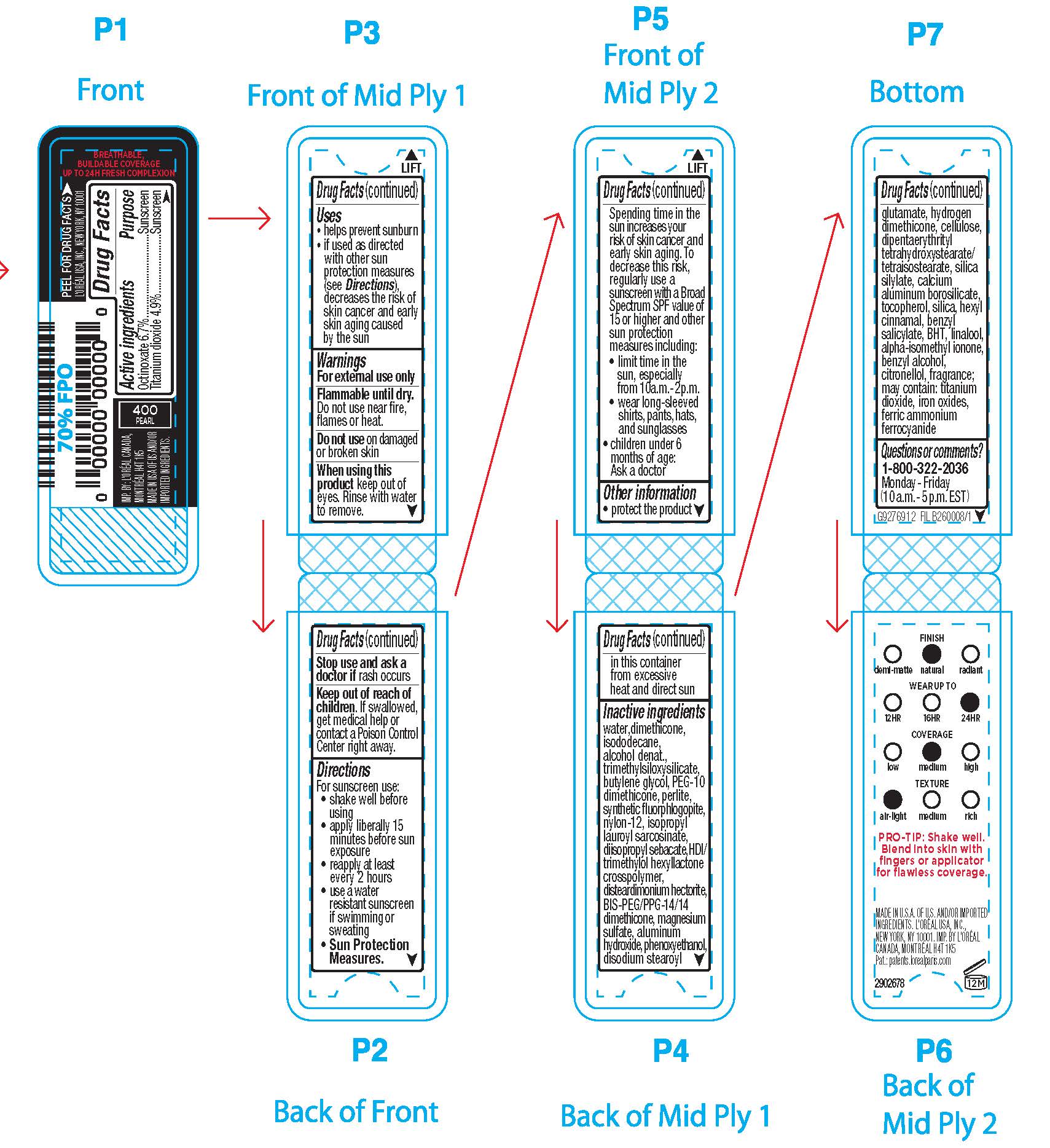
- Active ingredients
- Purpose
- Uses
- Warnings
- Flammable until dry.
- Do not use
- When using this product
- Stop use and ask a doctor if
- Keep out of reach of children.
-
Directions
For sunscreen use:
● shake well
● apply liberally 15 minutes before sun exposure
● reapply at least every 2 hours
● use a water resistant sunscreen if swimming or sweating
● Sun Protection Measures. Spending time in the sun increases your risk of skin cancer and early skin aging. To decrease this risk, regularly use a sunscreen with a Broad Spectrum SPF value of 15 or higher and other sun protection measures including:
● limit time in the sun, especially from 10 a.m. – 2 p.m.
● wear long-sleeved shirts, pants, hats, and sunglasses
● children under 6 months of age: Ask a doctor
- Other information
-
Inactive ingredients
water, dimethicone, isododecane, alcohol denat., trimethylsiloxysilicate, butylene glycol, PEG-10 dimethicone, perlite, synthetic fluorphlogopite, nylon-12, isopropyl lauroyl sarcosinate, diisopropyl sebacate, disteardimonium hectorite, HDI/trimethylol hexyllactone crosspolymer, bis-PEG/PPG-14/14 dimethicone, magnesium sulfate, aluminum hydroxide, phenoxyethanol, disodium stearoyl glutamate, hydrogen dimethicone, dipentaerythrityl tetrahydroxystearate/tetraisostearate, fragrance, silica silylate, acrylonitrile/methyl methacrylate/vinylidene chloride copolymer, tocopherol, hexyl cinnamal, silica, isobutane, benzyl salicylate, linalool, alpha-isomethyl ionone, benzyl alcohol, BHT, citronellol, may contain: titanium dioxide, iron oxides, ferric ammonium ferrocyanide
- Questions or comments?
- PRINCIPAL DISPLAY PANEL
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
LOREAL PARIS INFALLIBLE UP TO 24 H FRESH WEAR FOUNDATION BROAD SPECTRUM SPF 25 SUNSCREEN
octinoxate and titanium dioxide liquidProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:49967-009 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength OCTINOXATE (UNII: 4Y5P7MUD51) (OCTINOXATE - UNII:4Y5P7MUD51) OCTINOXATE 67 mg in 1 mL TITANIUM DIOXIDE (UNII: 15FIX9V2JP) (TITANIUM DIOXIDE - UNII:15FIX9V2JP) TITANIUM DIOXIDE 36 mg in 1 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) DIMETHICONE (UNII: 92RU3N3Y1O) ISODODECANE (UNII: A8289P68Y2) ALCOHOL (UNII: 3K9958V90M) TRIMETHYLSILOXYSILICATE (M/Q 0.6-0.8) (UNII: 5041RX63GN) BUTYLENE GLYCOL (UNII: 3XUS85K0RA) PEG-10 DIMETHICONE (600 CST) (UNII: 8PR7V1SVM0) PERLITE (UNII: 0SG101ZGK9) MAGNESIUM POTASSIUM ALUMINOSILICATE FLUORIDE (UNII: YK3DC63Y5M) NYLON-12 (UNII: 446U8J075B) ISOPROPYL LAUROYL SARCOSINATE (UNII: LYR06W430J) DIISOPROPYL SEBACATE (UNII: J8T3X564IH) DISTEARDIMONIUM HECTORITE (UNII: X687XDK09L) HEXAMETHYLENE DIISOCYANATE/TRIMETHYLOL HEXYLLACTONE CROSSPOLYMER (UNII: WB5K9Y35Y9) BIS-PEG/PPG-14/14 DIMETHICONE (UNII: X2I70H0QJE) MAGNESIUM SULFATE, UNSPECIFIED FORM (UNII: DE08037SAB) ALUMINUM HYDROXIDE (UNII: 5QB0T2IUN0) PHENOXYETHANOL (UNII: HIE492ZZ3T) DISODIUM STEAROYL GLUTAMATE (UNII: 45ASM2L11M) HYDROGEN DIMETHICONE (13 CST) (UNII: 4QGR4P2YOI) DIPENTAERYTHRITYL TETRAHYDROXYSTEARATE/TETRAISOSTEARATE (UNII: 230K0823CE) TOCOPHEROL (UNII: R0ZB2556P8) .ALPHA.-HEXYLCINNAMALDEHYDE (UNII: 7X6O37OK2I) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) ISOBUTANE (UNII: BXR49TP611) BENZYL SALICYLATE (UNII: WAO5MNK9TU) LINALOOL, (+/-)- (UNII: D81QY6I88E) ISOMETHYL-.ALPHA.-IONONE (UNII: 9XP4LC555B) BENZYL ALCOHOL (UNII: LKG8494WBH) BUTYLATED HYDROXYTOLUENE (UNII: 1P9D0Z171K) .BETA.-CITRONELLOL, (R)- (UNII: P01OUT964K) FERRIC OXIDE RED (UNII: 1K09F3G675) FERRIC AMMONIUM FERROCYANIDE (UNII: 9R0NVI936I) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:49967-009-01 30 mL in 1 BOTTLE; Type 0: Not a Combination Product 10/01/2018 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M020 10/01/2018 Labeler - L'Oreal USA Products Inc (002136794) Establishment Name Address ID/FEI Business Operations L'Oreal USA, Inc. 624244349 manufacture(49967-009)