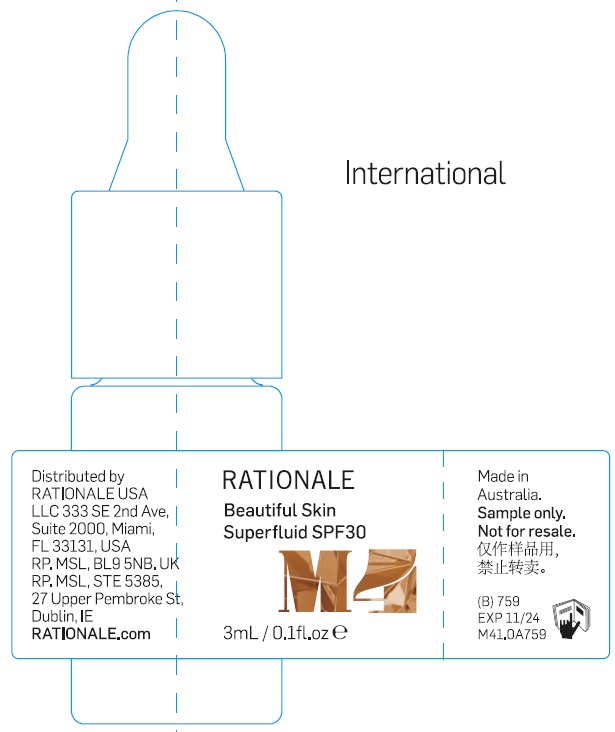
Label: BEAUTIFUL SKIN SUPERFLUID BROAD SPECTRUM SPF30 M4- zinc oxide emulsion
- NDC Code(s): 83108-040-00, 83108-040-03
- Packager: Rationale Group Pty Ltd
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated November 29, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Drug Facts
- Active Ingredient
- Uses
- Warnings
-
DirectionsMM
- Apply liberally 15 minutes before sun exposure.
- Reapply
- At least every 2 hours
- Use a water resistant sunscreen if swimming or sweating.
- Spending time in the sun increases your risk of skin cancer and early skin ageing. To decrease this risk, regularly use a sunscreen with a Broad Spectrum SPF value of 15 or higher and other sun protection measures including: Sun Protection Measures.
- Limit time in the sun, especially from 10 a.m.- 2p.m.
- Wear long sleeved shirts, pants, hats and sunglasses.
- Children under 6 months of age: Ask a doctor.
- Other Information
-
Inactive ingredients
Undecane, Aqua (Water), Coco-Caprylate, Caprylic/Capric Triglyceride, Tridecane, Butyloctyl Salicylate, Lauryl PEG-10 Tris(Trimethylsiloxy)silylethyl Dimethicone, Polyhydroxystearic Acid, Argania Spinosa Kernel Oil, Tocopheryl Acetate, Triacontanyl PVP, Dipropylene Glycol, Methyl Methacrylate Crosspolymer, Pentylene Glycol, Ethylhexyl Methoxycrylene, Sodium Palmitoyl Proline, Propanediol, CI 77891 (Titanium Dioxide), Silica, Butylene Glycol, Bis-Ethylhexyl Hydroxydimethoxy Benzylmalonate, Boron Nitride, Sodium Chloride, Polyurethane-79, Polyurethane-62, C12-15 Alkyl Benzoate, CI 77492 (Iron Oxides), Isopropyl Palmitate, Mica, Bisabolol, 1,2-Hexanediol, Sodium Potassium Aluminum Silicate, Limonene, Panthenol, Lecithin, Tocopherol, CI 77491 (Iron Oxides), Citrus Aurantium Dulcis (Orange) Oil, CI 77499 (Iron Oxides), Caprylhydroxamic Acid, Trideceth-10, Triethoxycaprylylsilane, Tin Oxide, Citrus Aurantium Bergamia (Bergamot) Leaf Oil, Juniperus Mexicana Oil, Pelargonium Graveolens Oil, Citrus Aurantium Amara (Bitter Orange) Flower Oil, Eugenia Caryophyllus (Clove) Leaf Oil, Ceteareth-25, Helianthus Annuus (Sunflower) Seed Oil, Melanin, Cyclopentasiloxane, Citrus Aurantium Bergamia (Bergamot) Fruit Oil, Citrus Grandis (Grapefruit) Peel Oil, Citrus Limon (Lemon) Peel Oil, Litsea Cubeba Fruit Oil, Citral, Glycerin, PEG/PPG-18/18 Dimethicone, Cetyl Alcohol, Magnesium Aspartate, Zinc Gluconate, Nymphaea Alba Flower Extract, Linalool, Glycine, Behenic Acid, Ceramide NP, Cholesterol, Ceramide NS, Serine, Glutamic Acid, Aspartic Acid, Leucine, Ethylhexylglycerin, Ceramide AP, Ceramide EOP, Ceramide EOS, Copper Gluconate, Alanine, Lysine, Arginine, Tyrosine, Phenylalanine, Proline, Threonine, Valine, Isoleucine, Caprooyl Phytosphingosine, Caprooyl Sphingosine, Phenoxyethanol, Sodium Hyaluronate, Histidine, Acetyl Hexapeptide-1.
- Package Labeling:30ml
- Package Labeling:3ml
-
INGREDIENTS AND APPEARANCE
BEAUTIFUL SKIN SUPERFLUID BROAD SPECTRUM SPF30 M4
zinc oxide emulsionProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:83108-040 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ZINC OXIDE (UNII: SOI2LOH54Z) (ZINC CATION - UNII:13S1S8SF37) ZINC CATION 159 mg in 1 mL Inactive Ingredients Ingredient Name Strength CITRAL (UNII: T7EU0O9VPP) GLYCERIN (UNII: PDC6A3C0OX) CETYL ALCOHOL (UNII: 936JST6JCN) MAGNESIUM ASPARTATE (UNII: R17X820ROL) ZINC GLUCONATE (UNII: U6WSN5SQ1Z) NYMPHAEA ALBA FLOWER (UNII: 40KQ7Q535O) LINALOOL, (+/-)- (UNII: D81QY6I88E) SORBITAN MONOOLEATE (UNII: 06XEA2VD56) GLYCINE (UNII: TE7660XO1C) BEHENIC ACID (UNII: H390488X0A) CERAMIDE NP (UNII: 4370DF050B) CHOLESTEROL (UNII: 97C5T2UQ7J) CERAMIDE NG (UNII: C04977SRJ5) SERINE (UNII: 452VLY9402) GLUTAMIC ACID (UNII: 3KX376GY7L) ASPARTIC ACID (UNII: 30KYC7MIAI) LEUCINE (UNII: GMW67QNF9C) ETHYLHEXYLGLYCERIN (UNII: 147D247K3P) CERAMIDE AP (UNII: F1X8L2B00J) CERAMIDE EOS (UNII: CR0J8RN66K) UNDECANE (UNII: JV0QT00NUE) WATER (UNII: 059QF0KO0R) COCO-CAPRYLATE (UNII: 4828G836N6) MEDIUM-CHAIN TRIGLYCERIDES (UNII: C9H2L21V7U) TRIDECANE (UNII: A3LZF0L939) BUTYLOCTYL SALICYLATE (UNII: 2EH13UN8D3) ARGAN OIL (UNII: 4V59G5UW9X) ALKYL (C12-15) BENZOATE (UNII: A9EJ3J61HQ) FERRIC OXIDE YELLOW (UNII: EX438O2MRT) .ALPHA.-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) TRICONTANYL POVIDONE (UNII: N0SS3Q238D) DIPROPYLENE GLYCOL (UNII: E107L85C40) PENTYLENE GLYCOL (UNII: 50C1307PZG) SODIUM PALMITOYL PROLINE (UNII: 64L053FRFO) ETHYLHEXYL METHOXYCRYLENE (UNII: S3KFG6Q5X8) PROPANEDIOL (UNII: 5965N8W85T) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) BUTYLENE GLYCOL (UNII: 3XUS85K0RA) BIS-ETHYLHEXYL HYDROXYDIMETHOXY BENZYLMALONATE (UNII: 7D4Q5YJ8NV) FERRIC OXIDE RED (UNII: 1K09F3G675) BORON NITRIDE (UNII: 2U4T60A6YD) SODIUM CHLORIDE (UNII: 451W47IQ8X) POLYURETHANE-62 (UNII: TBK645J3J8) ISOPROPYL PALMITATE (UNII: 8CRQ2TH63M) MICA (UNII: V8A1AW0880) LEVOMENOL (UNII: 24WE03BX2T) 1,2-HEXANEDIOL (UNII: TR046Y3K1G) TRIETHOXYCAPRYLYLSILANE (UNII: LDC331P08E) FERROSOFERRIC OXIDE (UNII: XM0M87F357) LIMONENE, (+)- (UNII: GFD7C86Q1W) OCTYLDODECANOL (UNII: 461N1O614Y) PANTHENOL (UNII: WV9CM0O67Z) CYCLOMETHICONE 5 (UNII: 0THT5PCI0R) TOCOPHEROL (UNII: R0ZB2556P8) FD&C BLUE NO. 1 (UNII: H3R47K3TBD) ORANGE OIL, COLD PRESSED (UNII: AKN3KSD11B) CAPRYLHYDROXAMIC ACID (UNII: UPY805K99W) TRIDECETH-10 (UNII: G624N6MSBA) PEG/PPG-18/18 DIMETHICONE (UNII: 9H0AO7T794) CITRUS BERGAMIA LEAF OIL (UNII: ZVY8741I1V) JUNIPERUS DEPPEANA WOOD OIL (UNII: 4739QA5686) GERANIUM OIL, ALGERIAN TYPE (UNII: 5Q1I94P4WG) CITRUS AURANTIUM FLOWER OIL (UNII: D4BGE91OXH) CLOVE LEAF OIL (UNII: VCA5491KVF) STANNIC OXIDE (UNII: KM7N50LOS6) CETEARETH-25 (UNII: 8FA93U5T67) SUNFLOWER OIL (UNII: 3W1JG795YI) CITRUS MAXIMA FRUIT RIND OIL (UNII: 8U3877WD44) LEMON OIL, COLD PRESSED (UNII: I9GRO824LL) LITSEA OIL (UNII: 2XIW34BN6O) COPPER GLUCONATE (UNII: RV823G6G67) ALANINE (UNII: OF5P57N2ZX) LYSINE (UNII: K3Z4F929H6) ARGININE (UNII: 94ZLA3W45F) TYROSINE (UNII: 42HK56048U) PHENYLALANINE (UNII: 47E5O17Y3R) PROLINE (UNII: 9DLQ4CIU6V) THREONINE (UNII: 2ZD004190S) VALINE (UNII: HG18B9YRS7) ISOLEUCINE (UNII: 04Y7590D77) CAPROOYL PHYTOSPHINGOSINE (UNII: 2FD4Y5XL2L) N-HEXANOYLSPHINGOSINE (UNII: 038753E78J) PHENOXYETHANOL (UNII: HIE492ZZ3T) HYALURONATE SODIUM (UNII: YSE9PPT4TH) HISTIDINE (UNII: 4QD397987E) ACETYL HEXAPEPTIDE-1 (UNII: 49ZWR266MZ) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:83108-040-00 1 in 1 CARTON 01/29/2023 1 30 mL in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product 2 NDC:83108-040-03 3 mL in 1 BOTTLE, DROPPER; Type 0: Not a Combination Product 01/29/2023 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M020 01/29/2023 Labeler - Rationale Group Pty Ltd (756927393)