Label: PURGENLABS ACETAMINOPHEN- actaminophen liquid
- NDC Code(s): 80513-230-08
- Packager: Advanced Rx LLC
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated June 28, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
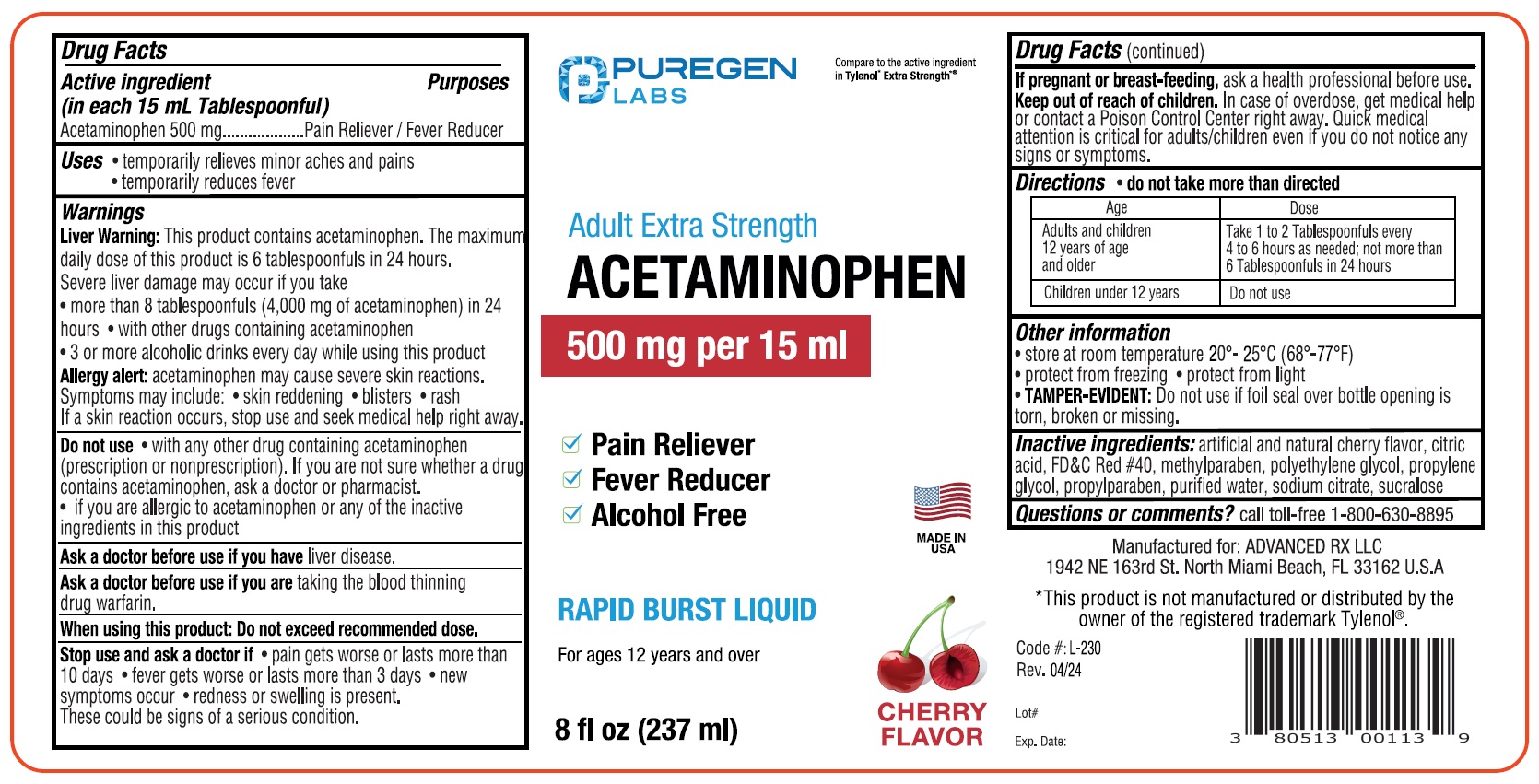
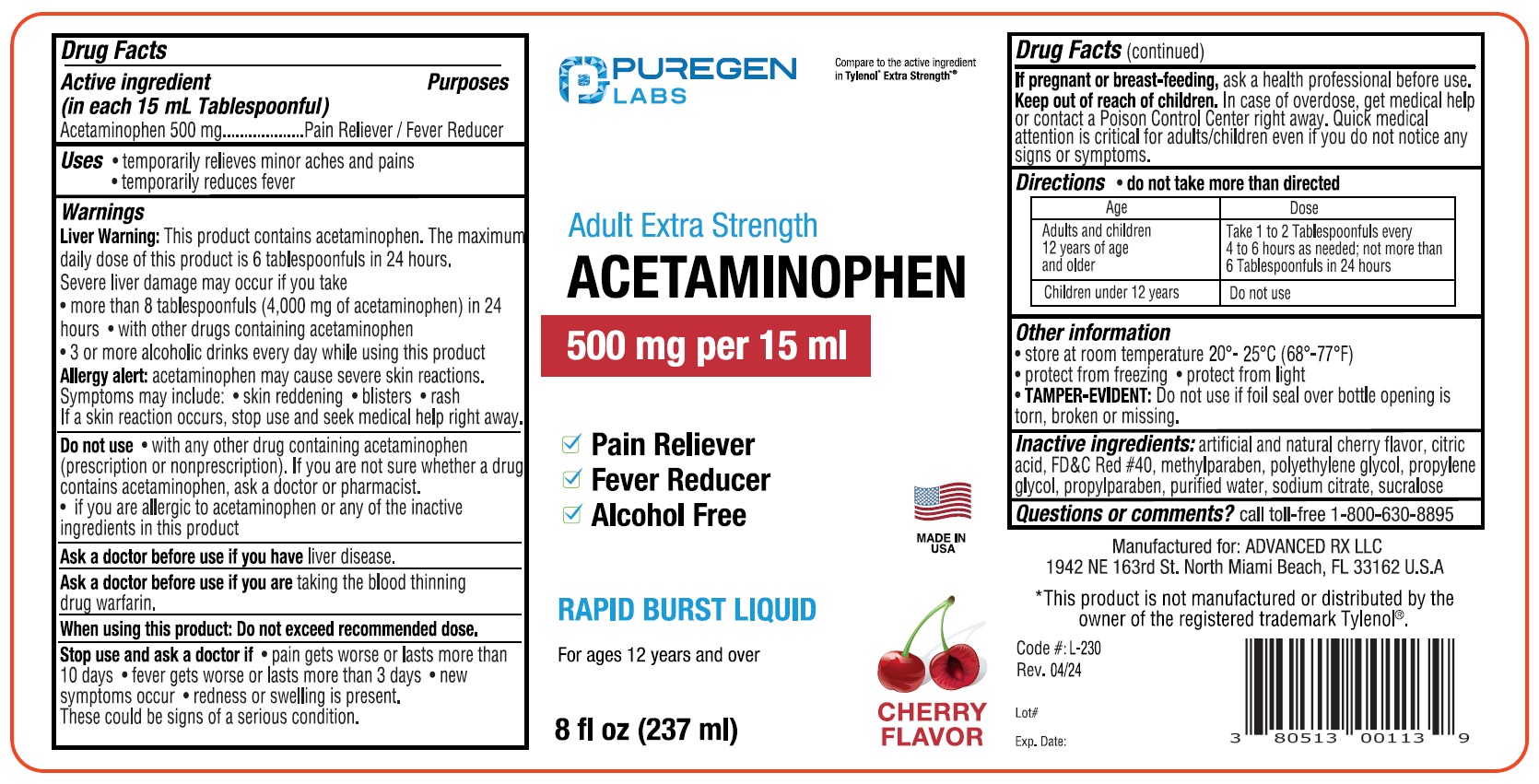
- ACTIVE INGREDIENT
- PURPOSE
- INDICATIONS & USAGE
-
WARNINGS
Warnings
Liver Warning:This product contains acetaminophen. The maximurr
daily dose of fhis product is 6 tablespoonfuls in 24 hours.
Severe liver damage may occur if you take
• more than 8 tablespoonfuls (4,000 mg of acetaminophen) in 24
hours • with other drugs containing acetaminophen
• 3 or more alcoholic drinks every day while using this productAllergy alert acetaminophen may cause severe skin reactions.
Symptoms may include: • skin reddening • blisters • rash
If a skin reaction occurs, stop use and seek medical help right away. -
DO NOT USE
Do not use • with any other drug containing acetaminophen
(prescription or nonprescription). If you are not sure whether a drug
contains acetaminophen, ask a octor or pharmacist.
• if you are allergic to acetaminophen or any of the inactive
ingredients in this productAsk a doctor before use ff rou have liver disease.
Ask a doctor before use ff you are taking the blood thinning
drug wartarin.
When using this product Do not exceed recommended dose. - STOP USE
- PREGNANCY OR BREAST FEEDING
- KEEP OUT OF REACH OF CHILDREN
- DOSAGE & ADMINISTRATION
- INACTIVE INGREDIENT
- QUESTIONS
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
PURGENLABS ACETAMINOPHEN
actaminophen liquidProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:80513-230 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ACETAMINOPHEN (UNII: 362O9ITL9D) (ACETAMINOPHEN - UNII:362O9ITL9D) ACETAMINOPHEN 500 mg in 15 mL Inactive Ingredients Ingredient Name Strength ANHYDROUS CITRIC ACID (UNII: XF417D3PSL) FD&C RED NO. 40 (UNII: WZB9127XOA) METHYLPARABEN (UNII: A2I8C7HI9T) POLYETHYLENE GLYCOL, UNSPECIFIED (UNII: 3WJQ0SDW1A) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) PROPYLPARABEN (UNII: Z8IX2SC1OH) WATER (UNII: 059QF0KO0R) SODIUM CITRATE (UNII: 1Q73Q2JULR) SUCRALOSE (UNII: 96K6UQ3ZD4) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:80513-230-08 1 in 1 CARTON 06/01/2024 1 237 mL in 1 BOTTLE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M012 06/01/2024 Labeler - Advanced Rx LLC (042795108)