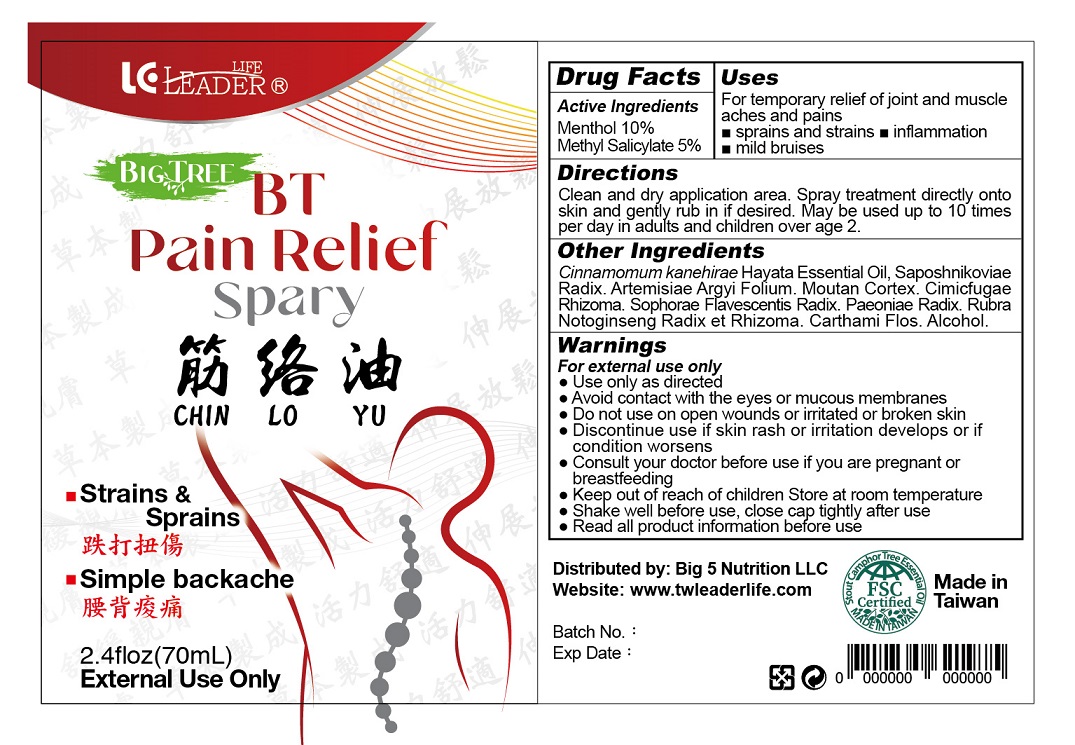
Label: BT PAIN RELIEF- menthol, methyl salicylate spray
- NDC Code(s): 82198-0012-1
- Packager: Big 5 Nutrition LLC
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated November 21, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Drug Facts
- Active Ingredients
- Purpose
- Uses
- Warnings
- KEEP OUT OF REACH OF CHILDREN
- Directions
- Other Information
- Inactive Ingredients
- Questions or Comments?
- SPL UNCLASSIFIED SECTION
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
BT PAIN RELIEF
menthol, methyl salicylate sprayProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:82198-0012 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength MENTHOL, UNSPECIFIED FORM (UNII: L7T10EIP3A) (MENTHOL - UNII:L7T10EIP3A) MENTHOL, UNSPECIFIED FORM 100 mg in 1 mL METHYL SALICYLATE (UNII: LAV5U5022Y) (SALICYLIC ACID - UNII:O414PZ4LPZ) METHYL SALICYLATE 50 mg in 1 mL Inactive Ingredients Ingredient Name Strength SOPHORA FLAVESCENS ROOT (UNII: IYR6K8KQ5K) PAEONIA LACTIFLORA ROOT (UNII: 3Z3866YW6P) PANAX NOTOGINSENG ROOT (UNII: GQX1C1175U) SAFFLOWER (UNII: 4VBL71TY4Y) ALCOHOL (UNII: 3K9958V90M) SAPOSHNIKOVIA DIVARICATA ROOT (UNII: 8H84LFK2QD) ARTEMISIA ARGYI LEAF (UNII: 2JYC99Q0WZ) PAEONIA X SUFFRUTICOSA ROOT BARK (UNII: BUG255FE7X) ACTAEA CIMICIFUGA ROOT (UNII: 65J267BAZT) Product Characteristics Color brown (Light Brown) Score Shape Size Flavor Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:82198-0012-1 70 mL in 1 BOTTLE; Type 0: Not a Combination Product 01/01/2024 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M017 01/01/2024 Labeler - Big 5 Nutrition LLC (114574559) Establishment Name Address ID/FEI Business Operations TAIWAN SHUENN-AN BIOTECHNOLOGY PHARMACEUTICAL CO., LTD. 656348265 manufacture(82198-0012) , label(82198-0012) , pack(82198-0012)