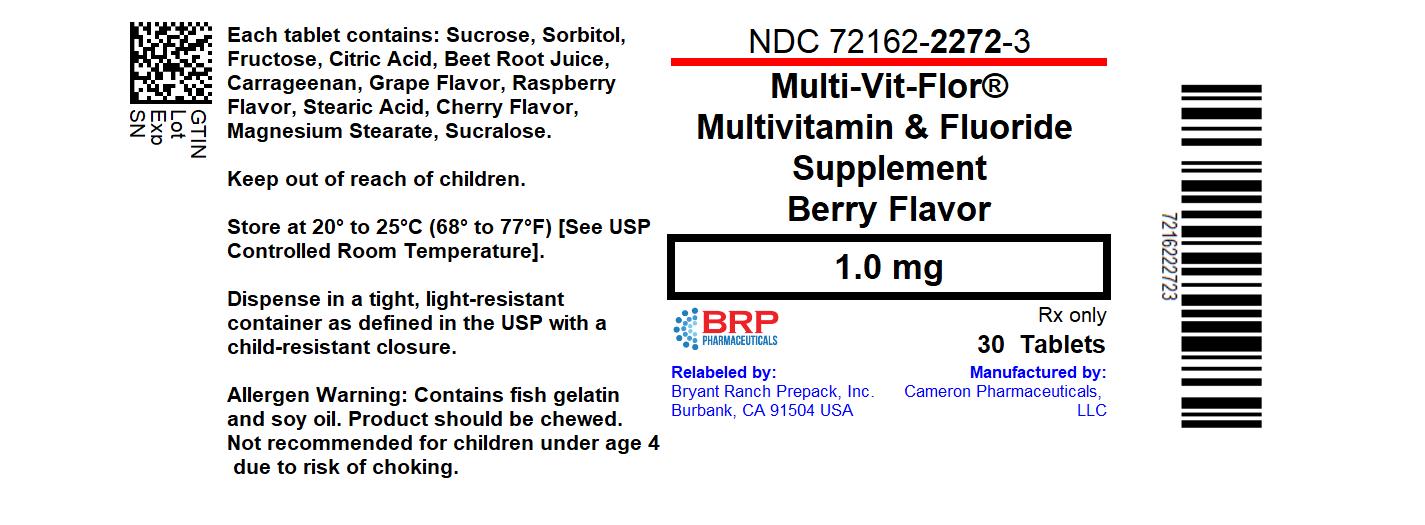
Label: MULTI-VIT-FLOR- sodium fluoride, vitamin a acetate, sodium ascorbate, cholecalciferol, .alpha.-tocopherol, d-, thiamine hydrochloride, riboflavin, niacinamide, pyridoxine hydrochloride, levomefolate calcium, and cyanocobalamin tablet, chewable
- NDC Code(s): 72162-2272-3
- Packager: Bryant Ranch Prepack
- This is a repackaged label.
- Source NDC Code(s): 42494-432
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: unapproved drug other
DISCLAIMER: This drug has not been found by FDA to be safe and effective, and this labeling has not been approved by FDA. For further information about unapproved drugs, click here.
Drug Label Information
Updated April 30, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- SPL UNCLASSIFIED SECTION
-
DESCRIPTION
Multi-Vit-Flor
(Multivitamin with 0.25 mg, 0.5 mg and 1 mg of Fluoride) Chewable Tablet
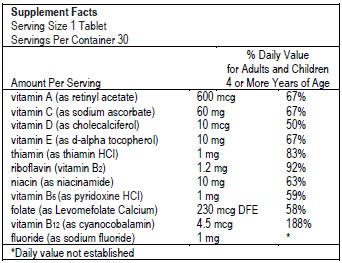
Multivitamin and Fluoride SupplementSupplement Facts
Serving Size 1 Tablet
Servings Per Container 30Amount Per Serving % Daily Value for Adults and Children 4 or More Years of Age - *
- Daily value not established
vitamin A (as retinyl acetate) 600 mcg 600 mcg 600 mcg 67% vitamin C (as sodium ascorbate) 60 mg 60 mg 60 mg 67% vitamin D (as cholecalciferol) 10 mcg 10 mcg 10 mcg 50% vitamin E (as d-alpha-tocopherol) 10 mg 10 mg 10 mg 67% thiamin (as thiamin HCl) 1 mg 1 mg 1 mg 83% riboflavin (vitamin B2) 1.2 mg 1.2 mg 1.2 mg 92% niacin (as niacinamide) 10 mg 10 mg 10 mg 63% vitamin B6 (as pyridoxine HCl) 1 mg 1 mg 1 mg 59% folate (as Levomefolate Calcium) 230 mcg DFE 230 mcg DFE 230 mcg DFE 58% vitamin B12 (as cyanocobalamin) 4.5 mcg 4.5 mcg 4.5 mcg 188% fluoride (as sodium fluoride) 0.25 mg 0.5 mg 1 mg * Ingredients: Sucrose, Sorbitol, Fructose, Citric Acid, Beet Root Juice, Carrageenan, Grape Flavor, Raspberry Flavor, Stearic Acid, Cherry Flavor, Magnesium Stearate, Sucralose.
- Allergen Warning
- Caution
- How Supplied
- SPL UNCLASSIFIED SECTION
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
MULTI-VIT-FLOR
sodium fluoride, vitamin a acetate, sodium ascorbate, cholecalciferol, .alpha.-tocopherol, d-, thiamine hydrochloride, riboflavin, niacinamide, pyridoxine hydrochloride, levomefolate calcium, and cyanocobalamin tablet, chewableProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:72162-2272(NDC:42494-432) Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength SODIUM FLUORIDE (UNII: 8ZYQ1474W7) (FLUORIDE ION - UNII:Q80VPU408O) SODIUM FLUORIDE 1 mg VITAMIN A ACETATE (UNII: 3LE3D9D6OY) (VITAMIN A - UNII:81G40H8B0T) VITAMIN A 600 ug SODIUM ASCORBATE (UNII: S033EH8359) (ASCORBIC ACID - UNII:PQ6CK8PD0R) ASCORBIC ACID 60 mg CHOLECALCIFEROL (UNII: 1C6V77QF41) (CHOLECALCIFEROL - UNII:1C6V77QF41) CHOLECALCIFEROL 10 ug .ALPHA.-TOCOPHEROL, D- (UNII: N9PR3490H9) (.ALPHA.-TOCOPHEROL, D- - UNII:N9PR3490H9) .ALPHA.-TOCOPHEROL, D- 10 mg THIAMINE HYDROCHLORIDE (UNII: M572600E5P) (THIAMINE ION - UNII:4ABT0J945J) THIAMINE HYDROCHLORIDE 1 mg RIBOFLAVIN (UNII: TLM2976OFR) (RIBOFLAVIN - UNII:TLM2976OFR) RIBOFLAVIN 1.2 mg NIACINAMIDE (UNII: 25X51I8RD4) (NIACINAMIDE - UNII:25X51I8RD4) NIACINAMIDE 10 mg PYRIDOXINE HYDROCHLORIDE (UNII: 68Y4CF58BV) (PYRIDOXINE - UNII:KV2JZ1BI6Z) PYRIDOXINE 1 mg LEVOMEFOLATE CALCIUM (UNII: A9R10K3F2F) (LEVOMEFOLIC ACID - UNII:8S95DH25XC) LEVOMEFOLATE CALCIUM 230 ug CYANOCOBALAMIN (UNII: P6YC3EG204) (CYANOCOBALAMIN - UNII:P6YC3EG204) CYANOCOBALAMIN 4.5 ug Inactive Ingredients Ingredient Name Strength SUCROSE (UNII: C151H8M554) SORBITOL (UNII: 506T60A25R) FRUCTOSE (UNII: 6YSS42VSEV) CITRIC ACID MONOHYDRATE (UNII: 2968PHW8QP) BEET JUICE (UNII: IOZ32L9H3O) CARRAGEENAN (UNII: 5C69YCD2YJ) STEARIC ACID (UNII: 4ELV7Z65AP) MAGNESIUM STEARATE (UNII: 70097M6I30) SUCRALOSE (UNII: 96K6UQ3ZD4) Product Characteristics Color PINK Score no score Shape ROUND Size 10mm Flavor Imprint Code V3 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:72162-2272-3 30 in 1 BOTTLE; Type 0: Not a Combination Product 04/30/2024 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date Unapproved drug other 02/02/2022 Labeler - Bryant Ranch Prepack (171714327) Registrant - Bryant Ranch Prepack (171714327) Establishment Name Address ID/FEI Business Operations Bryant Ranch Prepack 171714327 REPACK(72162-2272) , RELABEL(72162-2272)