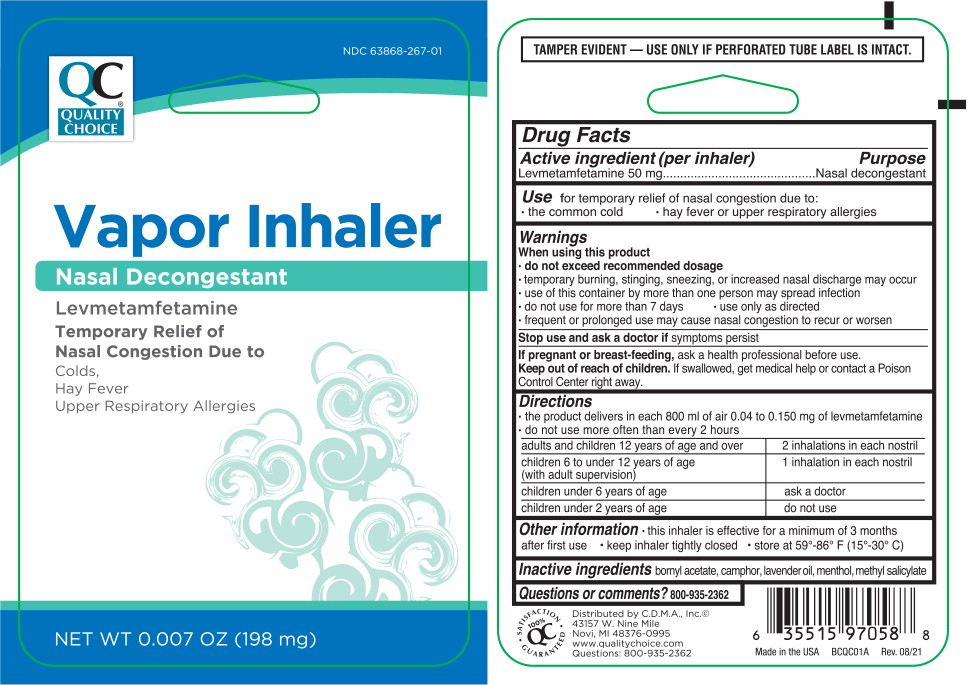
Label: VAPOR INHALER- levmetamfetamine nasal decongestant inhalant
- NDC Code(s): 63868-267-01
- Packager: C.D.M.A., Inc.
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: OTC monograph final
DISCLAIMER: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
Drug Label Information
Updated February 13, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Active ingredient (per inhaler)
- Purpose
- Use
-
Warnings
When using this product
- do not exceed recommended dosage
- temporary burning, stinging, sneezing, or increased nasal discharge may occur
- use of this container by more than one person may spread infection
-
Directions
- the product delivers in each 800 ml of air 0.04 to 0.150 mg of levmetamfetamine
- do not use more often than every 2 hours
adults and children 12 years of age and over 2 inhalations in each nostril children 6 to under 12 years of age (with adult supervision) 1 inhalation in each nostril children under 6 years of age ask a doctor children under 2 years of age do not use - Other information
- Inactive ingredients
- Questions or comments?
- Principal Display Panel – 198 mg Blister Pack Label
-
INGREDIENTS AND APPEARANCE
VAPOR INHALER
levmetamfetamine nasal decongestant inhalantProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:63868-267 Route of Administration NASAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Levmetamfetamine (UNII: Y24T9BT2Q2) (Levmetamfetamine - UNII:Y24T9BT2Q2) Levmetamfetamine 50 mg Inactive Ingredients Ingredient Name Strength Bornyl acetate (UNII: 213431586X) Camphor (Synthetic) (UNII: 5TJD82A1ET) Lavender oil (UNII: ZBP1YXW0H8) Menthol (UNII: L7T10EIP3A) Methyl Salicylate (UNII: LAV5U5022Y) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:63868-267-01 1 in 1 BLISTER PACK 01/01/2014 1 198 in 1 INHALER; Type 2: Prefilled Drug Delivery Device/System (syringe, patch, etc.) Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph final part341 01/01/2014 Labeler - C.D.M.A., Inc. (011920774) Registrant - Aphena Pharma Solutions MD, LLC (829739833) Establishment Name Address ID/FEI Business Operations Aphena Pharma Solutions MD, LLC 829739833 MANUFACTURE(63868-267)