Label: CROMOLYN SODIUM solution
- NDC Code(s): 69784-205-30, 69784-205-60
- Packager: Woodward Pharma Services LLC
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated July 29, 2022
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
DESCRIPTION
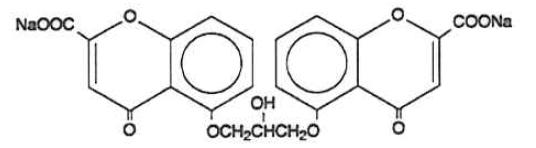
The active ingredient of cromolyn sodium inhalation solution USP is cromolyn sodium, USP. It is an inhaled anti-inflammatory agent for the preventive management of asthma. Cromolyn sodium, USP is chemically designated as disodium 5,5'- [(2-hydroxytrimethylene)dioxy] bis [4-oxo-4H-1-benzopyran-2-carboxylate]. The molecular formula is C23H14Na2O11; the molecular weight is 512.34. Cromolyn sodium, USP is a water-soluble, odorless, white, hydrated crystalline powder. It is tasteless at first, but leaves a slightly bitter aftertaste. Cromolyn sodium inhalation solution USP is clear, colorless to pale yellow, sterile and has a target pH of 5.5.
The structural formula is:
Each 2 mL vial for oral inhalation use only contains 20 mg cromolyn sodium, USP in water for injection, USP.
-
CLINICAL PHARMACOLOGY
In vitro and in vivo animal studies have shown that cromolyn sodium inhibits sensitized mast cell degranulation which occurs after exposure to specific antigens. Cromolyn sodium acts by inhibiting the release of mediators from mast cells. Studies show that cromolyn sodium indirectly blocks calcium ions from entering the mast cell, thereby preventing mediator release.
Cromolyn sodium inhibits both the immediate and non-immediate bronchoconstrictive reactions to inhaled antigen. Cromolyn sodium also attenuates bronchospasm caused by exercise, toluene diisocyanate, aspirin, cold air, sulfur dioxide, and environmental pollutants.
Cromolyn sodium has no intrinsic bronchodilator or antihistamine activity.
After administration by inhalation, approximately 8% of the total cromolyn sodium dose administered is absorbed and rapidly excreted unchanged, approximately equally divided between urine and bile. The remainder of the dose is either exhaled or deposited in the oropharynx, swallowed and excreted via the alimentary tract.
-
INDICATIONS AND USAGE
Cromolyn sodium inhalation solution USP is a prophylactic agent indicated in the management of patients with bronchial asthma.
In patients whose symptoms are sufficiently frequent to require a continuous program of medication, cromolyn sodium inhalation solution USP is given by inhalation on a regular daily basis (see DOSAGE AND ADMINISTRATION). The effect of cromolyn sodium is usually evident after several weeks of treatment, although some patients show an almost immediate response.
In patients who develop acute bronchoconstriction in response to exposure to exercise, toluene diisocyanate, environmental pollutants, etc., cromolyn sodium should be given shortly before exposure to the precipitating factor (see DOSAGE AND ADMINISTRATION).
- CONTRAINDICATIONS
- WARNINGS
-
PRECAUTIONS
General
Occasionally, patients may experience cough and/or bronchospasm following inhalation of cromolyn sodium. At times, patients who develop bronchospasm may not be able to continue cromolyn sodium administration despite prior bronchodilator administration. Rarely, very severe bronchospasm has been encountered.
Symptoms of asthma may recur if cromolyn sodium is reduced below the recommended dosage or discontinued.
Information for Patients
Cromolyn sodium is to be taken as directed by the physician. Because it is preventive medication, it may take up to four weeks before the patient experiences maximum benefit.
Cromolyn sodium should be used in a power-driven nebulizer with an adequate airflow rate equipped with a suitable face mask or mouthpiece.
Drug stability and safety of cromolyn sodium inhalation solution when mixed with other drugs in a nebulizer have not been established.
For additional information, see the accompanying leaflet entitled Living a Full Life with Asthma.
Carcinogenesis, Mutagenesis, and Impairment of Fertility
Long-term studies of cromolyn sodium in mice (12 months intraperitoneal administration at doses up to 150 mg/kg three days per week), hamsters (intraperitoneal administration at doses up to 53 mg/kg three days per week for 15 weeks followed by 17.5 mg/kg three days per week for 37 weeks), and rats (18 months subcutaneous treatment at doses up to 75 mg/kg six days per week) showed no neoplastic effects. These doses correspond to approximately 1.0, 0.3, and 2 times, respectively, the maximum recommended human daily inhalation dose on a mg/m2 basis.
Cromolyn sodium showed no mutagenic potential in Ames Salmonella/microsome plate assays, mitotic gene conversion in Saccharomyces cerevisiae and in an in vitro cytogenetic study in human peripheral lymphocytes.
No evidence of impaired fertility was shown in laboratory reproduction studies conducted subcutaneously in rats at the highest doses tested, 175 mg/kg/day in males and 100 mg/kg/day in females. These doses are approximately 18 and 10 times, respectively, the maximum recommended adult human daily inhalation dose on a mg/m2 basis.
Pregnancy
Teratogenic Effects
Pregnancy Category B
Reproduction studies with cromolyn sodium administered subcutaneously to pregnant mice and rats at maximum daily doses of 540 mg/kg and 164 mg/kg, respectively, and intravenously to rabbits at a maximum daily dose of 485 mg/kg produced no evidence of fetal malformations. These doses represent approximately 27, 17, and 98 times, respectively, the maximum recommended adult human daily inhalation dose on a mg/m2 basis. Adverse fetal effects (increased resorption and decreased fetal weight) were noted only at the very high parenteral doses that produced maternal toxicity. There are, however, no adequate and well-controlled studies in pregnant women.
Because animal reproduction studies are not always predictive of human response, this drug should be used during pregnancy only if clearly needed.
Drug Interaction During Pregnancy
Cromolyn sodium and isoproterenol were studied following subcutaneous injections in pregnant mice. Cromolyn sodium alone in doses up to 540 mg/kg (approximately 27 times the maximum recommended adult human daily inhalation dose on a mg/m2 basis) did not cause significant increases in resorptions or major malformations. Isoproterenol alone at a dose of 2.7 mg/kg (approximately 7 times the maximum recommended adult human daily inhalation dose on a mg/m2 basis) increased both resorptions and malformations. The addition of cromolyn sodium (approximately 27 times the maximum recommended adult human daily inhalation dose on a mg/m2 basis) to isoproterenol (approximately 7 times the maximum recommended adult human daily inhalation dose on a mg/m2 basis) appears to have increased the incidence of both resorptions and malformations.
Nursing Mothers
It is not known whether this drug is excreted in human milk. Because many drugs are excreted in human milk, caution should be exercised when cromolyn sodium is administered to a nursing woman.
Pediatric Use
Safety and effectiveness in pediatric patients below the age of 2 years have not been established.
Geriatric Use
Clinical studies of cromolyn sodium inhalation solution did not include sufficient numbers of subjects aged 65 and over to determine whether they respond differently from younger subjects. Other reported clinical experience has not identified differences in responses between the elderly and younger patients.
-
ADVERSE REACTIONS
Clinical experience with the use of cromolyn sodium suggests that adverse reactions are rare events. The following adverse reactions have been associated with cromolyn sodium: cough, nasal congestion, nausea, sneezing, and wheezing.
Other reactions have been reported in clinical trials; however, a causal relationship could not be established: drowsiness, nasal itching, nose bleed, nose burning, serum sickness, and stomachache.
In addition, adverse reactions have been reported with cromolyn sodium for inhalation capsules. The most common side effects are associated with inhalation of the powder and include transient cough (1 in 5 patients) and mild wheezing (1 in 25 patients). These effects rarely require treatment or discontinuation of the drug.
Information on the incidence of adverse reactions to cromolyn sodium for inhalation capsules has been derived from U.S. postmarketing surveillance experience. The following adverse reactions attributed to cromolyn sodium, based upon recurrence following readministration, have been reported in less than 1 in 10,000 patients: laryngeal edema, swollen parotid gland, angioedema, bronchospasm, joint swelling and pain, dizziness, dysuria and urinary frequency, nausea, cough, wheezing, headache, nasal congestion, rash, urticaria and lacrimation.
Other adverse reactions have been reported in less than 1 in 100,000 patients, and it is unclear whether these are attributable to the drug: anaphylaxis, nephrosis, periarteritic vasculitis, pericarditis, peripheral neuritis, pulmonary infiltrates with eosinophilia, polymyositis, exfoliative dermatitis, hemoptysis, anemia, myalgia, hoarseness, photodermatitis, and vertigo.
Call your doctor for medical advice about side effects. You may report side effects to Woodward Pharma Services LLC at 1-888-514-4727 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
-
OVERDOSAGE
There is no clinical syndrome associated with an overdosage of cromolyn sodium. Acute toxicity testing in a wide variety of species has demonstrated that toxicity with cromolyn sodium occurs only with very high exposure levels, regardless of whether administration was parenteral, oral or by inhalation. Parenteral administration in mice, rats, guinea pigs, hamsters, and rabbits demonstrated a median lethal dose of approximately 4000 mg/kg. Intravenous administration in monkeys also indicated a similar pattern of toxicity. The highest dose administered by the oral route in rats and mice was 8000 mg/kg, (approximately 261 and 130 times, respectively, the maximum recommended human daily inhalation dose on a mg/m2 basis) and at this dose level no deaths occurred. By inhalation, even in long term studies, it proved impossible to achieve toxic dose levels of cromolyn sodium in a range of mammalian species.
-
DOSAGE AND ADMINISTRATION
For management of bronchial asthma in adults and pediatric patients (two years of age and over), the usual starting dosage is the contents of one vial administered by nebulization four times a day at regular intervals.
Drug stability and safety of cromolyn sodium inhalation solution when mixed with other drugs in a nebulizer have not been established.
Patients with chronic asthma should be advised that the effect of cromolyn sodium inhalation solution therapy is dependent upon its administration at regular intervals, as directed. Cromolyn sodium inhalation solution should be introduced into the patient's therapeutic regimen when the acute episode has been controlled, the airway has been cleared and the patient is able to inhale adequately.
For the prevention of acute bronchospasm which follows exercise or exposure to cold dry air, environmental agents (e.g., animal danders, toluene diisocyanate, pollutants), etc., the usual dose is the contents of one vial administered by nebulization shortly before exposure to the precipitating factor.
It should be emphasized to the patient that the drug is poorly absorbed when swallowed and is not effective by this route of administration.
For additional information, see the accompanying leaflet entitled “Living a Full Life with Asthma”.
Cromolyn Sodium Inhalation Solution Therapy in Relation to Other Treatments for Asthma
Non-steroidal agents
Cromolyn sodium inhalation solution should be added to the patient's existing treatment regimen (e.g., bronchodilators). When a clinical response to cromolyn sodium inhalation solution is evident, usually within two to four weeks, and if the asthma is under good control, an attempt may be made to decrease concomitant medication usage gradually.
If concomitant medications are eliminated or required on no more than a prn basis, the frequency of administration of cromolyn sodium inhalation solution may be titrated downward to the lowest level consistent with the desired effect. The usual decrease is from four to three vials per day. It is important that the dosage be reduced gradually to avoid exacerbation of asthma. It is emphasized that in patients whose dosage has been titrated to fewer than four vials per day, an increase in the dose of cromolyn sodium inhalation solution and the introduction of, or increase in, symptomatic medications may be needed if the patient's clinical condition deteriorates.
Corticosteroids
In patients chronically receiving corticosteroids for the management of bronchial asthma, the dosage should be maintained following the introduction of cromolyn sodium inhalation solution. If the patient improves, an attempt to decrease corticosteroids should be made. Even if the corticosteroid-dependent patient fails to show symptomatic improvement following cromolyn sodium inhalation solution administration, the potential to reduce corticosteroids may nonetheless be present. Thus, gradual tapering of corticosteroid dosage may be attempted. It is important that the dose be reduced slowly, maintaining close supervision of the patient to avoid an exacerbation of asthma.
It should be borne in mind that prolonged corticosteroid therapy frequently causes an impairment in the activity of the hypothalamic-pituitary-adrenal axis and a reduction in the size of the adrenal cortex. A potentially critical degree of impairment or insufficiency may persist asymptomatically for some time even after gradual discontinuation of adrenocortical steroids. Therefore, if a patient is subjected to significant stress, such as a severe asthmatic attack, surgery, trauma or severe illness while being treated or within one year (occasionally up to two years) after corticosteroid treatment has been terminated, consideration should be given to reinstituting corticosteroid therapy. When respiratory function is impaired, as may occur in severe exacerbation of asthma, a temporary increase in the amount of corticosteroids may be required to regain control of the patient's asthma.
It is particularly important that great care be exercised if, for any reason, cromolyn sodium inhalation solution is withdrawn in cases where its use has permitted a reduction in the maintenance dose of corticosteroids. In such cases, continued close supervision of the patient is essential since there may be sudden reappearance of severe manifestations of asthma which will require immediate therapy and possible reintroduction of corticosteroids.
-
HOW SUPPLIED
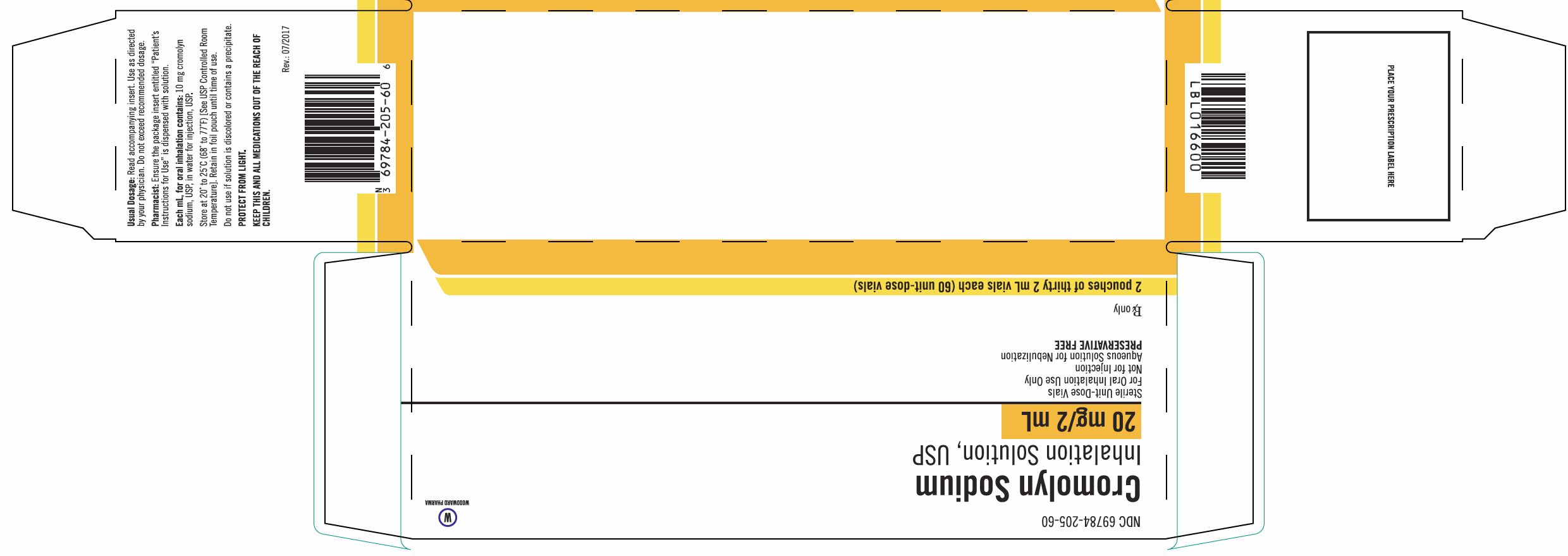
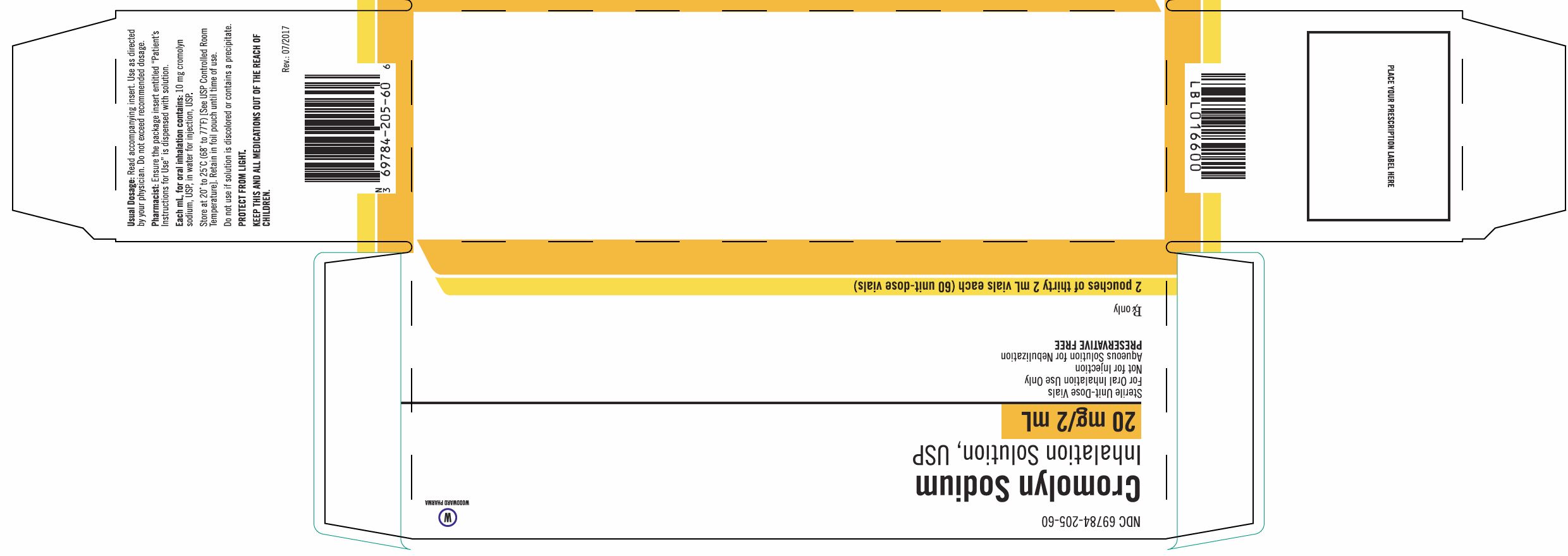
Cromolyn Sodium Inhalation Solution USP Unit-Dose 2 mL Vial is supplied as a colorless to pale yellow solution containing 20 mg cromolyn sodium, USP, in water for injection, USP, with 30 vials per foil pouch in a carton as listed below.
60 vials per carton (NDC 70556-102-60).
Each vial is made from a low density polyethylene (LDPE) resin.
Store at 20° to 25°C (68° to 77°F) [See USP Controlled Room Temperature]. Do not use if solution is discolored or contains a precipitate.
Retain in foil pouch until time of use.
PROTECT FROM LIGHT.
KEEP THIS AND ALL MEDICATIONS OUT OF THE REACH OF CHILDREN.
Marketed by:
Woodward Pharma Services LLC
Birmingham, MI 48009 -
LIVING A FULL LIFE WITH ASTHMA
PATIENT INSTRUCTIONS
CROMOLYN SODIUM INHALATION SOLUTION USP
You or your child may be among the millions of Americans who have asthma. For most patients, asthma need not limit your lifestyle, if you closely follow the asthma management plan your doctor provides you. Your doctor has given you this instruction sheet to help you learn more about asthma and ways to control it.
Instructions for the Use of
CROMOLYN SODIUM INHALATION SOLUTION USP
An aqueous solution for nebulization
NOT FOR INJECTION
For best results, follow these instructions exactly and observe Care and Storage directions.
METHOD OF ADMINISTRATION
Cromolyn sodium inhalation solution is recommended for use in a power driven nebulizer operated at an airflow rate of 6 to 8 liters per minute and equipped with a suitable face mask. Hand-operated nebulizers are not suitable for the administration of cromolyn sodium inhalation solution. Your doctor will advise on the choice of a suitable nebulizer and how it should be used. Do not use any appliance without consulting your doctor.
Drug stability and safety of cromolyn sodium inhalation solution when mixed with other drugs in a nebulizer have not been established.
DOSAGE
Nebulization should be carried out four times a day at regular intervals, or as directed by your doctor. Use the contents of a fresh vial each time.
INHALATION
Once the nebulizer has been assembled and contains cromolyn sodium inhalation solution, hold the mask close to the patient’s face and switch on the device. The patient should breathe in through the mouth and out through the nose in a normal, relaxed manner. Nebulization should take approximately five to ten minutes.
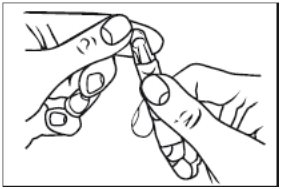
1. Remove a single unit-dose vial from strip (Figure 1).
Figure 1
2. Open the unit-dose vial by twisting off the tabbed top section (Figure 2).
Figure 2
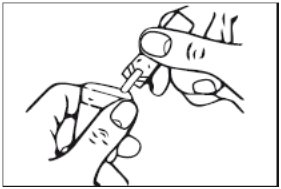
3. Squeeze the contents of the unit-dose vial into the solution container of your nebulizer (Figure 3).
Figure 3
Discard the empty unit-dose vial.
Marketed By:
Woodward Pharma Services LLC
Birmingham, MI 48009Rev.: 07/2017
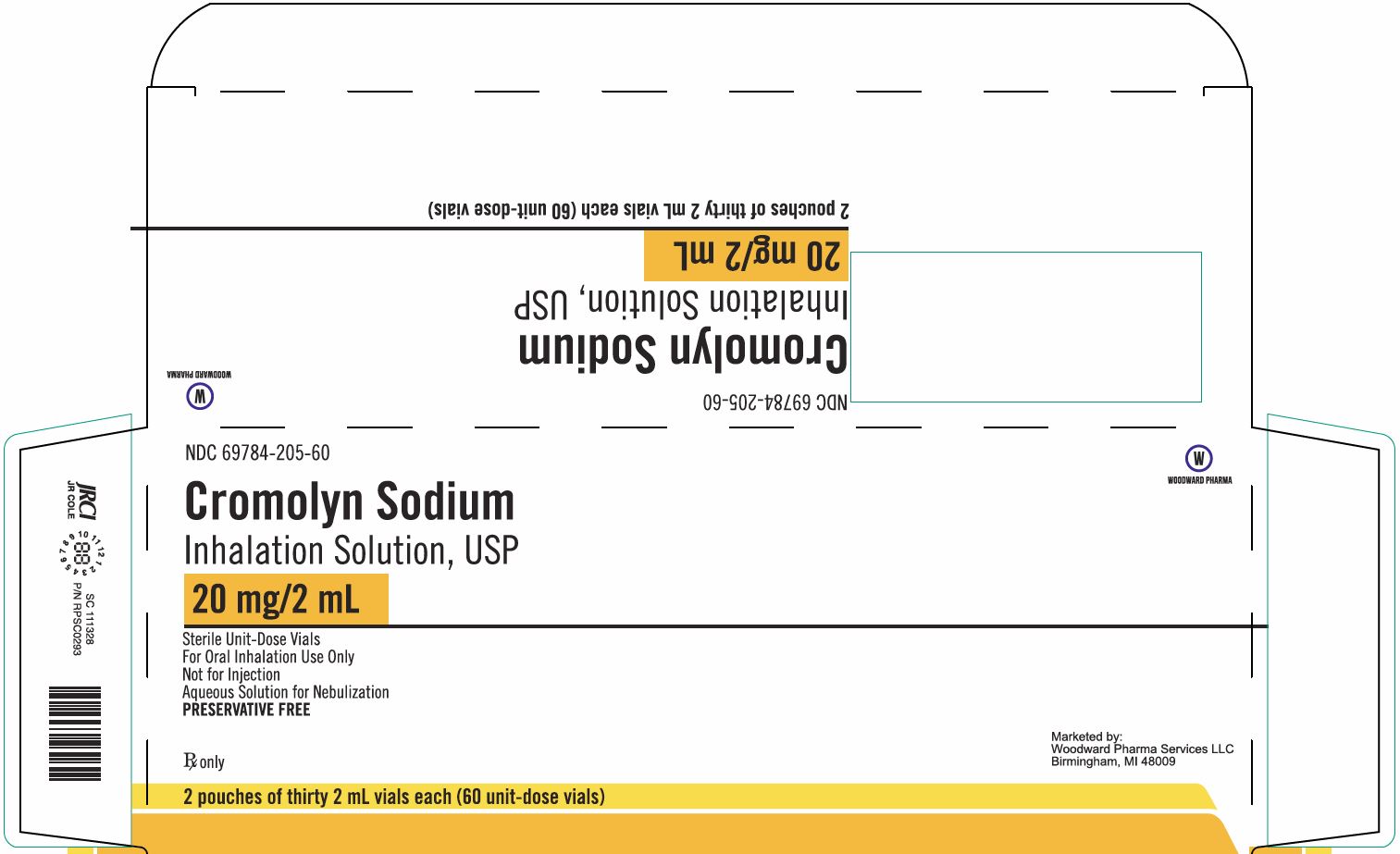
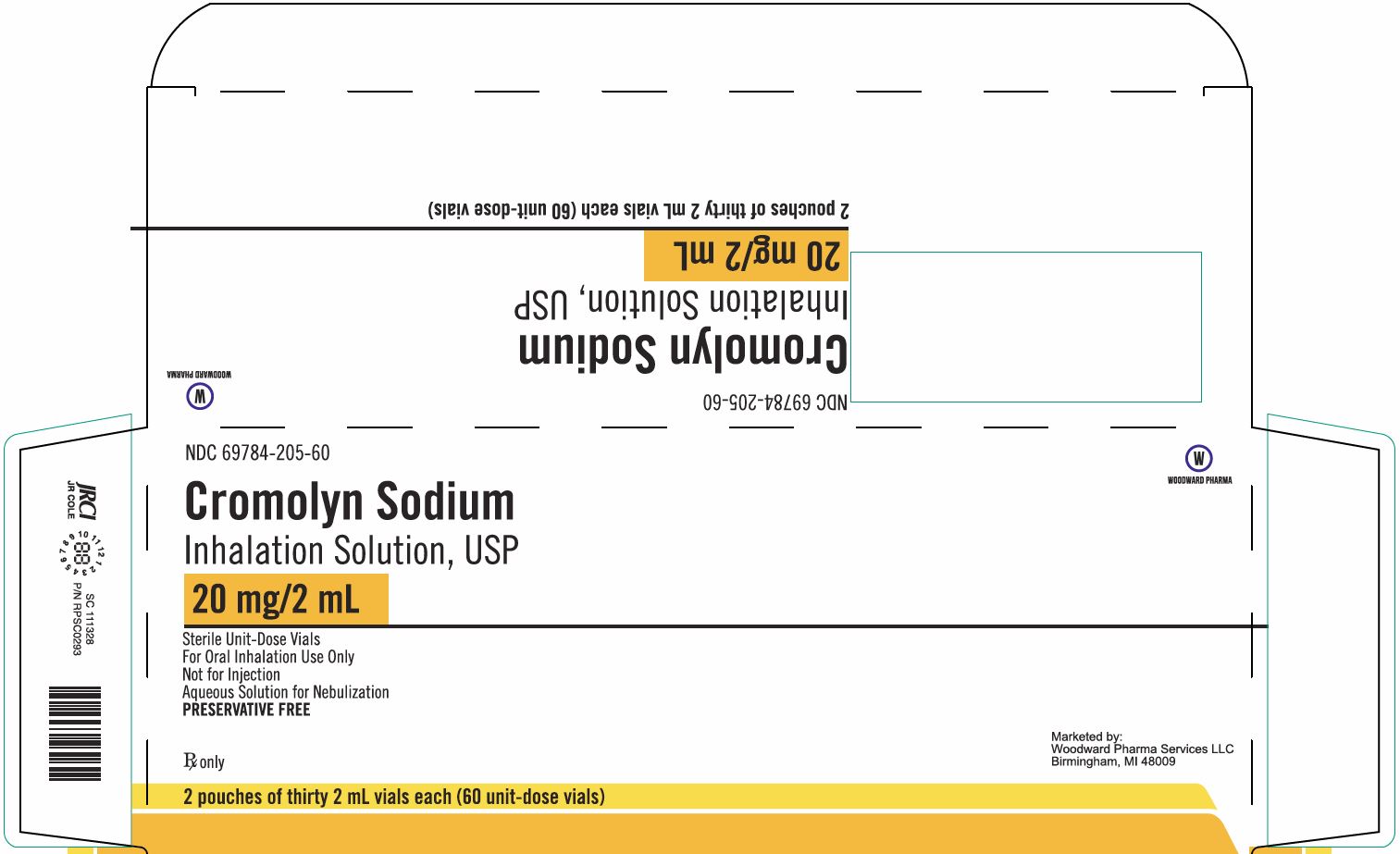
- Package/Label Display Panel, Part 1 of 2
-
Package/Label Display Panel, Part 2 of 2
Cromolyn Sodium Inhalation Solution 20 mg/2 mL 60 Unit-Dose Vials Carton Text
NDC 69784-205-60
Cromolyn Sodium
Inhalation Solution USP20 mg/2 mL
Sterile Unit-Dose Vials
For Oral Inhalation Use Only
Not for Injection
Aqueous Solution for Nebulization
PRESERVATIVE FREERx only
2 pouches of thirty 2 mL vials each (60 unit-dose vials)
-
INGREDIENTS AND APPEARANCE
CROMOLYN SODIUM
cromolyn sodium solutionProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:69784-205 Route of Administration INTRABRONCHIAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength CROMOLYN SODIUM (UNII: Q2WXR1I0PK) (CROMOLYN - UNII:Y0TK0FS77W) CROMOLYN SODIUM 20 mg in 2 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:69784-205-60 2 in 1 CARTON 10/16/2017 1 NDC:69784-205-30 30 in 1 POUCH 1 2 mL in 1 VIAL, SINGLE-DOSE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA209453 10/16/2017 Labeler - Woodward Pharma Services LLC (080406260) Registrant - Woodward Pharma Services LLC (080406260)