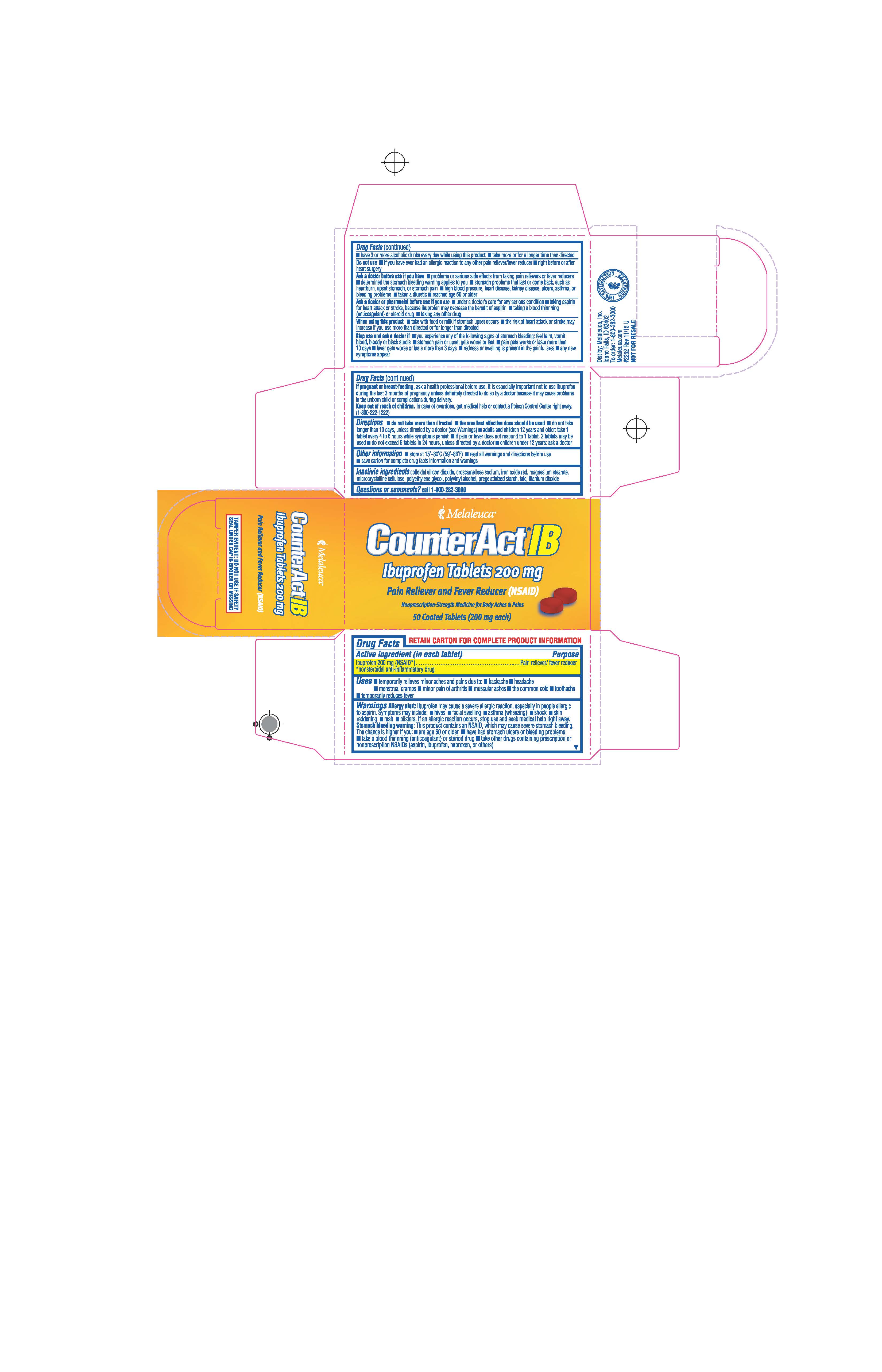
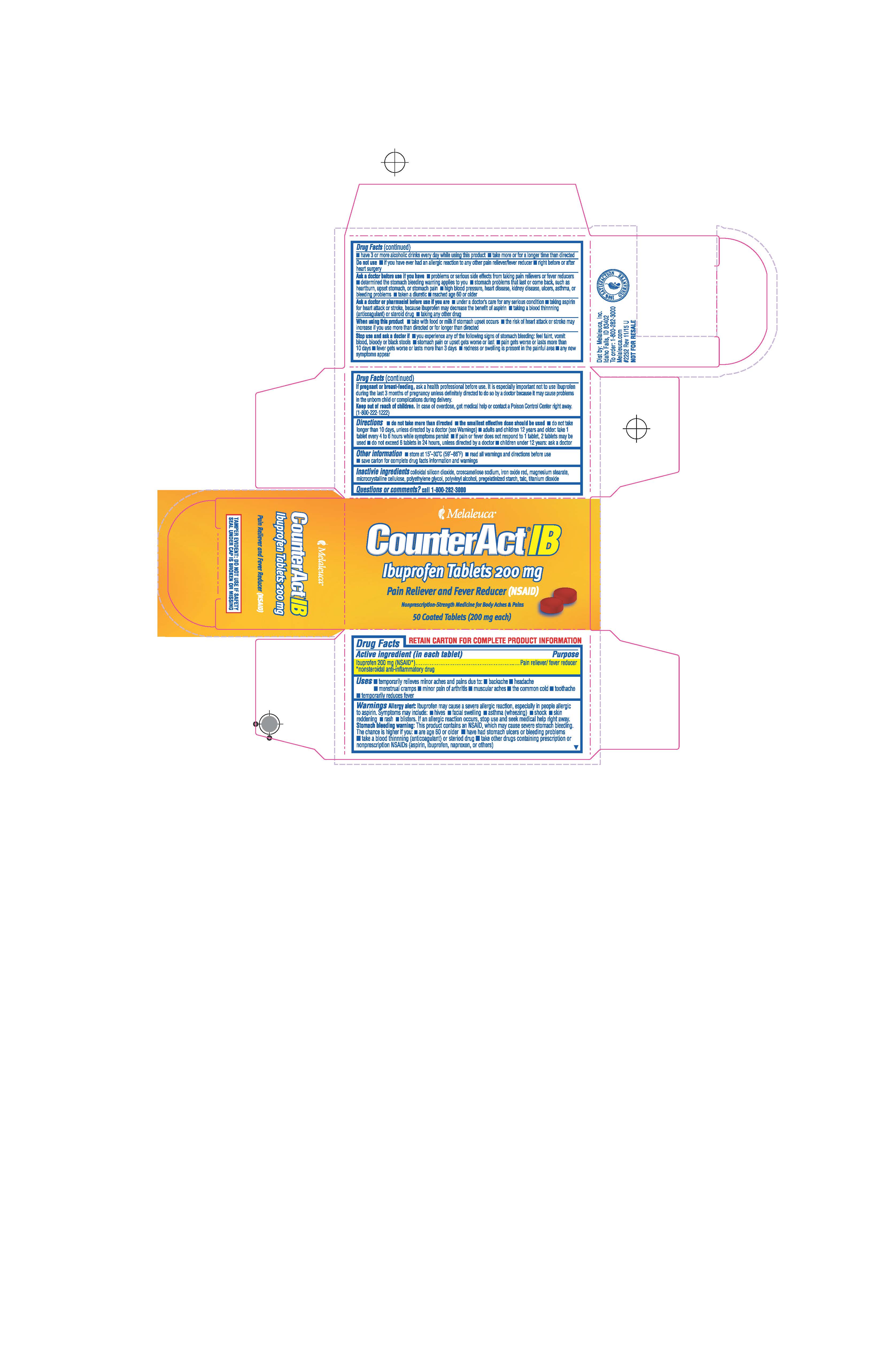
Label: COUNTERACT IB- ibuprofen tablet, coated
- NDC Code(s): 54473-308-50
- Packager: Melaleuca, Inc
- Category: HUMAN OTC DRUG LABEL
Drug Label Information
Updated January 11, 2023
If you are a healthcare professional or from the pharmaceutical industry please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- ACTIVE INGREDIENT
- PURPOSE
- INDICATIONS & USAGE
- WARNINGS
- DO NOT USE
-
ASK DOCTOR
Ask a doctor before use if
- you have problems or serious side effects from taking pain relievers or fever reducers
- the stomach bleeding warning applies to you
- you have a history of stomach problems such as heartburn
- you have high blood pressure, heart disease, liver cirrhosis, or kidney disease
- you have asthma
- you are taking a diuretic
-
ASK DOCTOR/PHARMACIST
Ask a doctor or pharmacist before use if you are
- taking aspirin for heart attack or stroke, because ibuprofen may decrease this benefit of aspirin
- under a doctor's care for any serious condition
- taking any other drug
- taking any other drug containing an NSAID (prescription or nonprescription)
- taking a blood thinning (anticoagulant) or steroid drug
- WHEN USING
-
STOP USE
Stop use and ask a doctor if
- you experience any of the following signs of bleeding:
-
- feel faint
- vomit blood
- have blood ro black stools
- have stomach pain that does not get better
- pain gets worse or lasts more than 10 days
- fever gets worse or lasts more than 3 days
- redness or swelling is present in the painful area
- any new symptoms appear
- PREGNANCY OR BREAST FEEDING
- KEEP OUT OF REACH OF CHILDREN
-
DOSAGE & ADMINISTRATION
Directions
- do not take more than directed
- the smallest effective dose should be used
Adults and children 12 years and older:
- take 1 tablet every 4 to 6 hours while symptoms persist
- if pain or fever does not respond to 1 tablet, 2 tablets may be used
- do not exceed 6 tablets in 24 hours unless directed by a doctor
- Children under 12 years:
-
- ask a doctor
- STORAGE AND HANDLING
- INACTIVE INGREDIENT
- QUESTIONS
- BOXED WARNING (What is this?)
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
COUNTERACT IB
ibuprofen tablet, coatedProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:54473-308 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength IBUPROFEN (UNII: WK2XYI10QM) (IBUPROFEN - UNII:WK2XYI10QM) IBUPROFEN 200 mg Inactive Ingredients Ingredient Name Strength CROSCARMELLOSE SODIUM (UNII: M28OL1HH48) TALC (UNII: 7SEV7J4R1U) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) HYPROMELLOSES (UNII: 3NXW29V3WO) FERRIC OXIDE RED (UNII: 1K09F3G675) MAGNESIUM STEARATE (UNII: 70097M6I30) CELLULOSE, MICROCRYSTALLINE (UNII: OP1R32D61U) POLYETHYLENE GLYCOL (UNII: 3WJQ0SDW1A) POLYVINYL ALCOHOL, UNSPECIFIED (UNII: 532B59J990) STARCH, CORN (UNII: O8232NY3SJ) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) Product Characteristics Color brown (Brown) Score no score Shape ROUND (Round) Size 10mm Flavor Imprint Code 114 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:54473-308-50 1 in 1 BOX 08/15/2019 1 50 in 1 BOTTLE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA091239 08/15/2019 Labeler - Melaleuca, Inc (139760102) Registrant - Melaleuca, Inc (139760102)