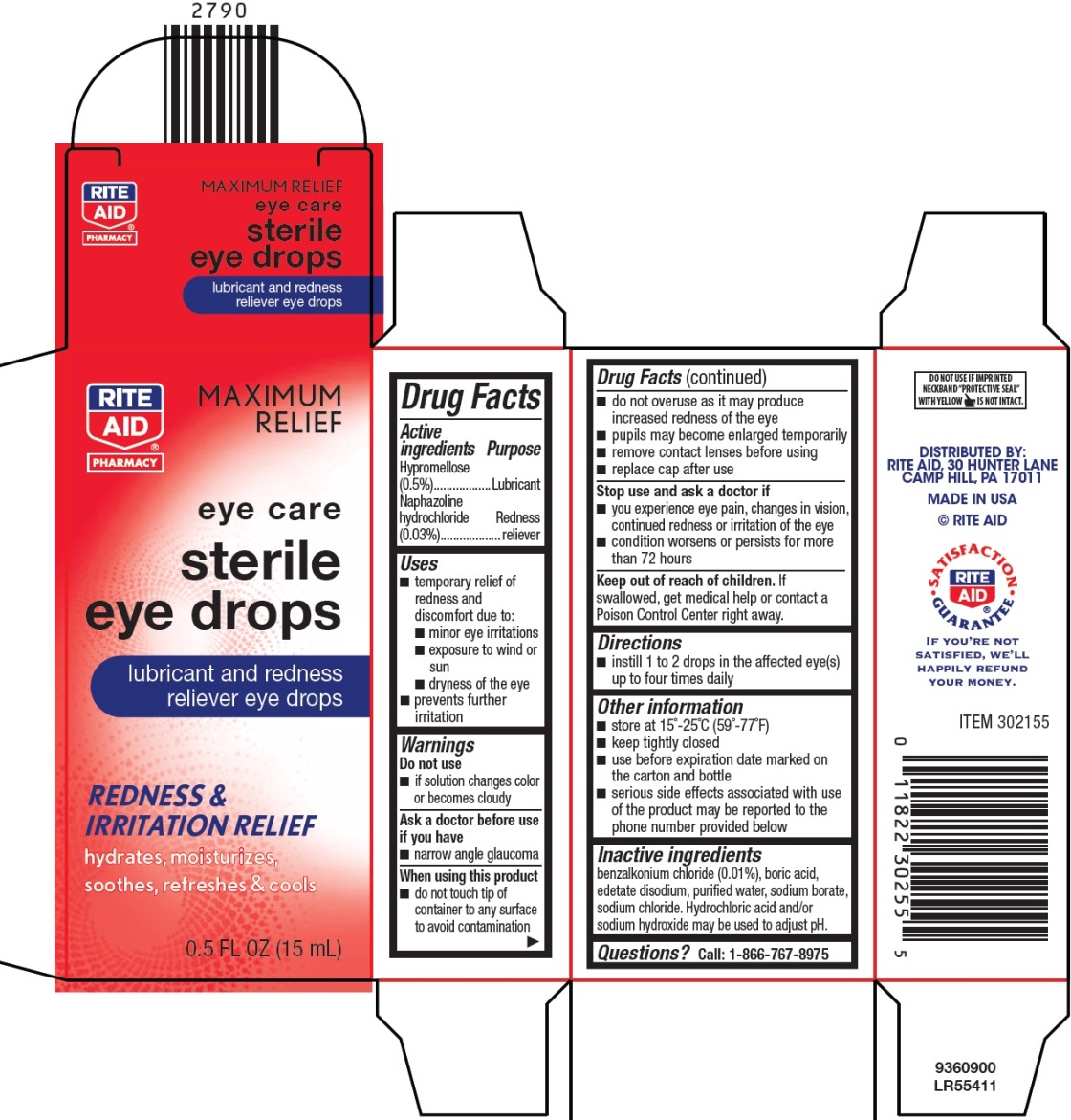
Label: LUBRICANT / REDNESS RELIEVER- naphazoline hydrochloride and hypromellose solution/ drops
-
Contains inactivated NDC Code(s)
NDC Code(s): 11822-3025-5 - Packager: Rite Aid Corporation
- Category: HUMAN OTC DRUG LABEL
DISCLAIMER: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
Drug Label Information
Updated December 4, 2018
If you are a healthcare professional or from the pharmaceutical industry please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Active ingredients
- Purpose
- Uses
-
Warnings
When using this product
- •
- do not touch tip of container to any surface to avoid contamination
- •
- do not overuse as it may produce increased redness of the eye
- •
- pupils may become enlarged temporarily
- •
- remove contact lenses before using
- •
- replace cap after use
- Directions
- Other information
- Inactive ingredients
- Questions?
- Package/Label Principal Display Panel
-
INGREDIENTS AND APPEARANCE
LUBRICANT / REDNESS RELIEVER
naphazoline hydrochloride and hypromellose solution/ dropsProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:11822-3025 Route of Administration OPHTHALMIC Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength NAPHAZOLINE HYDROCHLORIDE (UNII: MZ1131787D) (NAPHAZOLINE - UNII:H231GF11BV) NAPHAZOLINE HYDROCHLORIDE 5 mg in 1 mL HYPROMELLOSE, UNSPECIFIED (UNII: 3NXW29V3WO) (HYPROMELLOSE, UNSPECIFIED - UNII:3NXW29V3WO) HYPROMELLOSE, UNSPECIFIED 0.3 mg in 1 mL Inactive Ingredients Ingredient Name Strength BENZALKONIUM CHLORIDE (UNII: F5UM2KM3W7) BORIC ACID (UNII: R57ZHV85D4) EDETATE DISODIUM (UNII: 7FLD91C86K) WATER (UNII: 059QF0KO0R) SODIUM BORATE (UNII: 91MBZ8H3QO) SODIUM CHLORIDE (UNII: 451W47IQ8X) HYDROCHLORIC ACID (UNII: QTT17582CB) SODIUM HYDROXIDE (UNII: 55X04QC32I) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:11822-3025-5 1 in 1 CARTON 09/02/2010 1 15 mL in 1 BOTTLE, DROPPER; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph final part349 09/02/2010 Labeler - Rite Aid Corporation (014578892) Registrant - Bausch & Lomb Incorporated (196603781) Establishment Name Address ID/FEI Business Operations Bausch & Lomb Incorporated 114406598 MANUFACTURE(11822-3025)