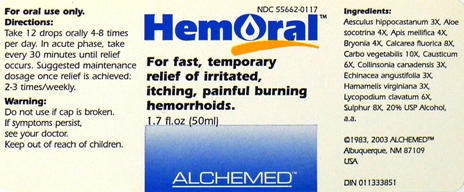
Label: HEMORAL- aesculus hippocastanum, aloe socotrina, apis mellifica, bryonia, calcarea fluorica, carbo vegetabilis, causticum, collinsonia canadensis, echinacea angustifolia, hamamelis virginiana, lycopodium clavatum, sulphur liquid
-
Contains inactivated NDC Code(s)
NDC Code(s): 55662-0117-1 - Packager: Alchemed
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: unapproved homeopathic
DISCLAIMER: This homeopathic product has not been evaluated by the Food and Drug Administration for safety or efficacy. FDA is not aware of scientific evidence to support homeopathy as effective.
Drug Label Information
Updated January 1, 2012
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Purpose:
- Usage and Dosage:
- DOSAGE & ADMINISTRATION
- Warnings:
- WARNINGS
- Active Ingredients:
- Inactive Ingredients:
- QUESTIONS
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
HEMORAL
aesculus hippocastanum, aloe socotrina, apis mellifica, bryonia, calcarea fluorica, carbo vegetabilis, causticum, collinsonia canadensis, echinacea angustifolia, hamamelis virginiana, lycopodium clavatum, sulphur liquidProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:55662-0117 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength HORSE CHESTNUT (UNII: 3C18L6RJAZ) (HORSE CHESTNUT - UNII:3C18L6RJAZ) HORSE CHESTNUT 3 [hp_X] in 1 mL ALOE (UNII: V5VD430YW9) (ALOE - UNII:V5VD430YW9) ALOE 4 [hp_X] in 1 mL APIS MELLIFERA (UNII: 7S82P3R43Z) (APIS MELLIFERA - UNII:7S82P3R43Z) APIS MELLIFERA 4 [hp_X] in 1 mL BRYONIA ALBA ROOT (UNII: T7J046YI2B) (BRYONIA ALBA ROOT - UNII:T7J046YI2B) BRYONIA ALBA ROOT 4 [hp_X] in 1 mL CALCIUM FLUORIDE (UNII: O3B55K4YKI) (CALCIUM FLUORIDE - UNII:O3B55K4YKI) CALCIUM FLUORIDE 8 [hp_X] in 1 mL ACTIVATED CHARCOAL (UNII: 2P3VWU3H10) (ACTIVATED CHARCOAL - UNII:2P3VWU3H10) ACTIVATED CHARCOAL 10 [hp_X] in 1 mL CAUSTICUM (UNII: DD5FO1WKFU) (CAUSTICUM - UNII:DD5FO1WKFU) CAUSTICUM 6 [hp_X] in 1 mL COLLINSONIA CANADENSIS ROOT (UNII: O2630F3XDR) (COLLINSONIA CANADENSIS ROOT - UNII:O2630F3XDR) COLLINSONIA CANADENSIS ROOT 3 [hp_X] in 1 mL ECHINACEA ANGUSTIFOLIA (UNII: VB06AV5US8) (ECHINACEA ANGUSTIFOLIA - UNII:VB06AV5US8) ECHINACEA ANGUSTIFOLIA 3 [hp_X] in 1 mL HAMAMELIS VIRGINIANA ROOT BARK/STEM BARK (UNII: T7S323PKJS) (HAMAMELIS VIRGINIANA ROOT BARK/STEM BARK - UNII:T7S323PKJS) HAMAMELIS VIRGINIANA ROOT BARK/STEM BARK 3 [hp_X] in 1 mL LYCOPODIUM CLAVATUM SPORE (UNII: C88X29Y479) (LYCOPODIUM CLAVATUM SPORE - UNII:C88X29Y479) LYCOPODIUM CLAVATUM SPORE 6 [hp_X] in 1 mL SULFUR (UNII: 70FD1KFU70) (SULFUR - UNII:70FD1KFU70) SULFUR 8 [hp_X] in 1 mL Inactive Ingredients Ingredient Name Strength ALCOHOL (UNII: 3K9958V90M) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:55662-0117-1 50 mL in 1 BOTTLE Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved homeopathic 06/01/2011 Labeler - Alchemed (957680150)