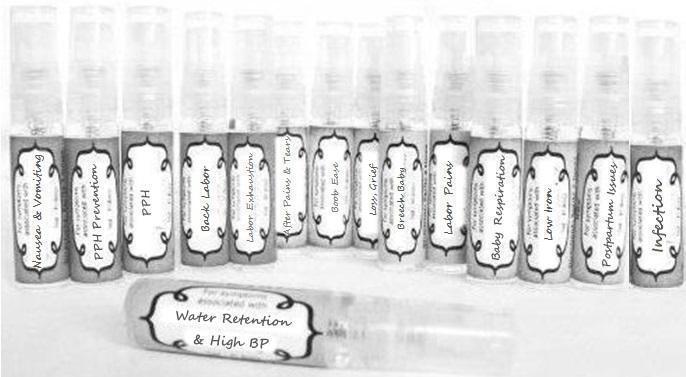
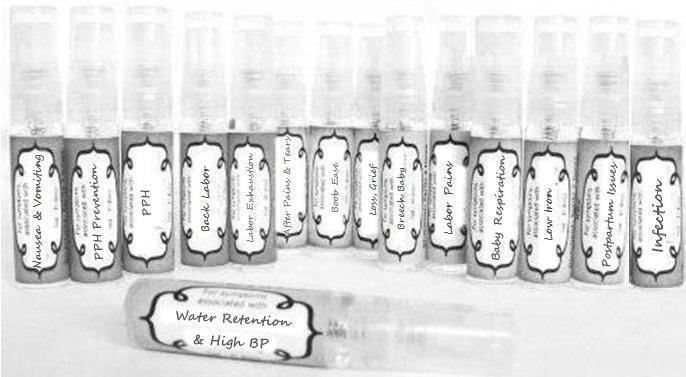
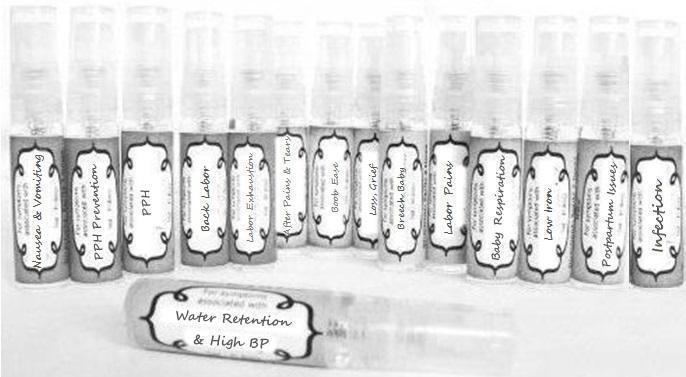
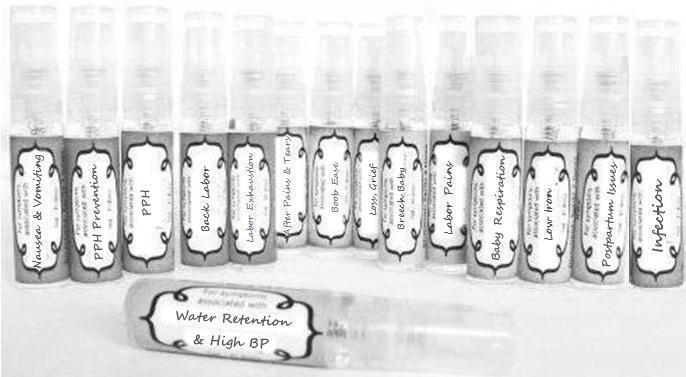
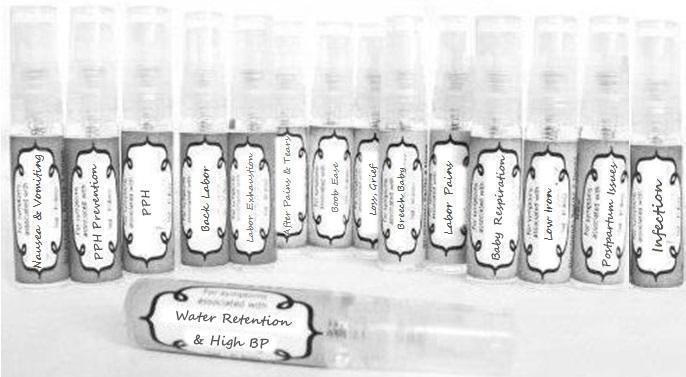
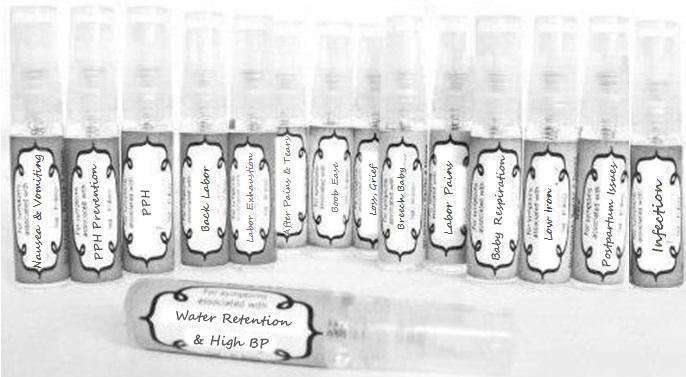
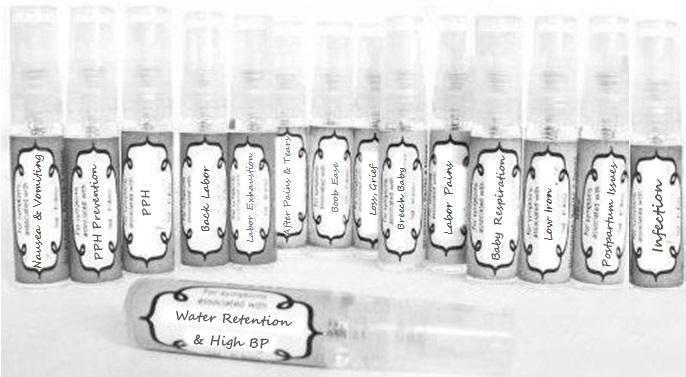
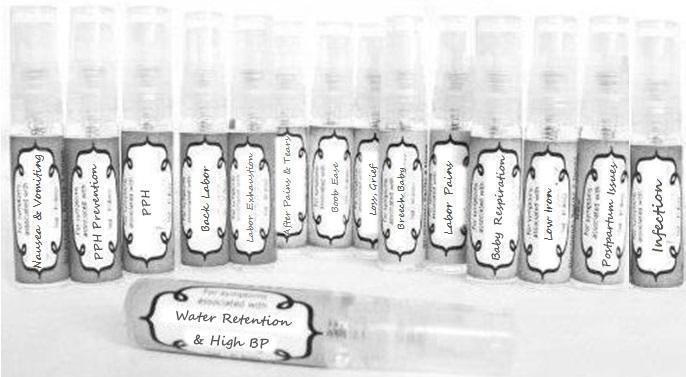
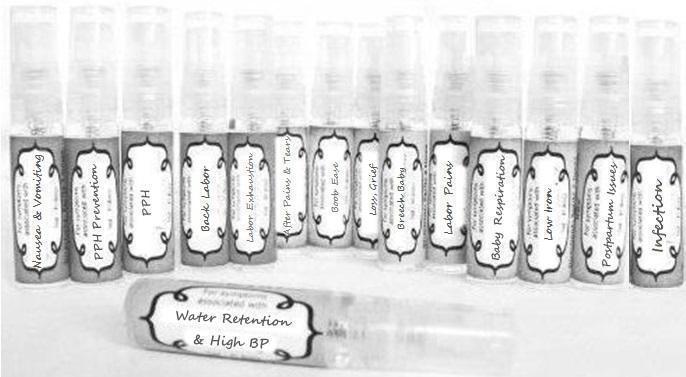
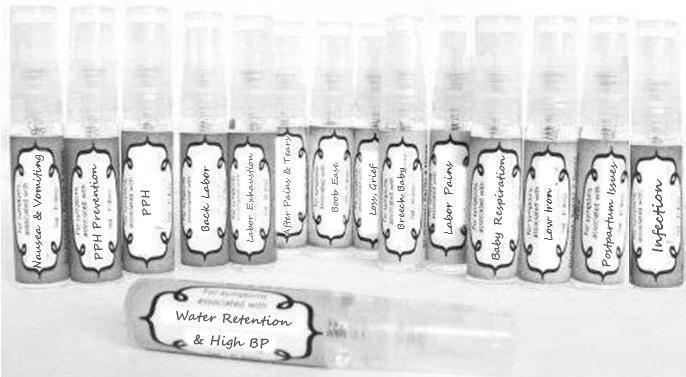
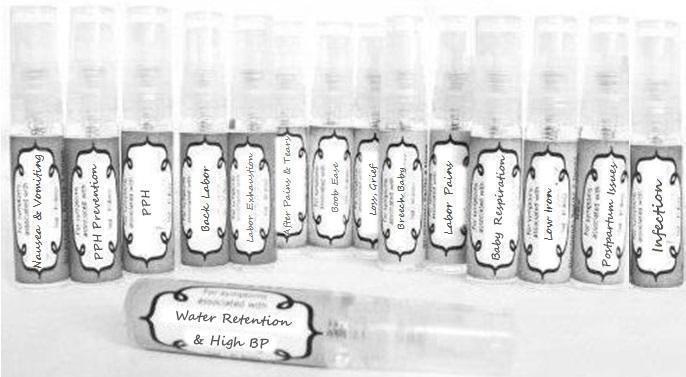
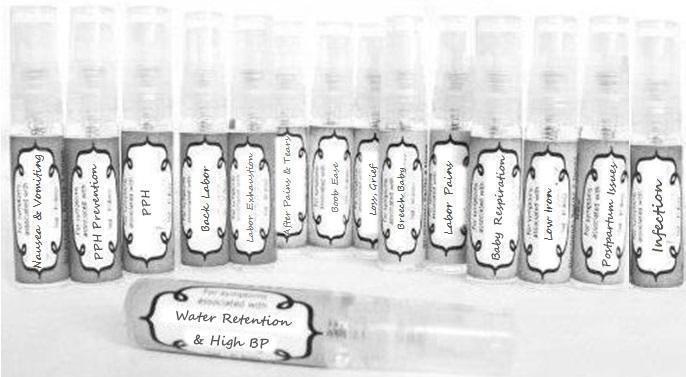
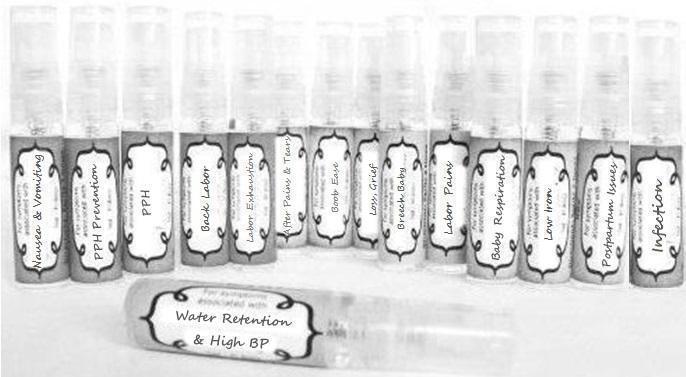
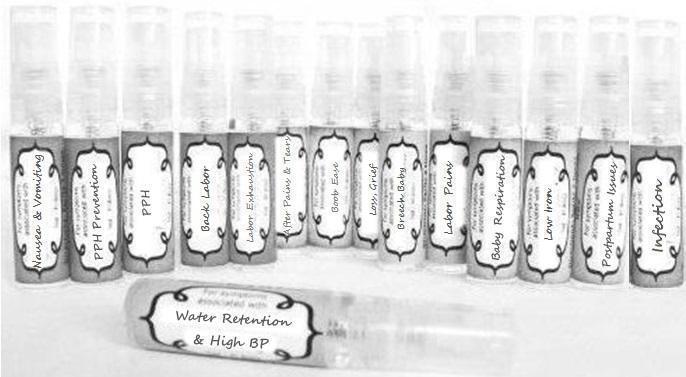
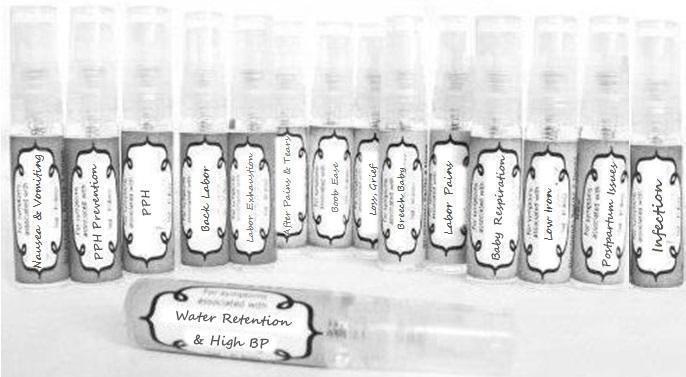
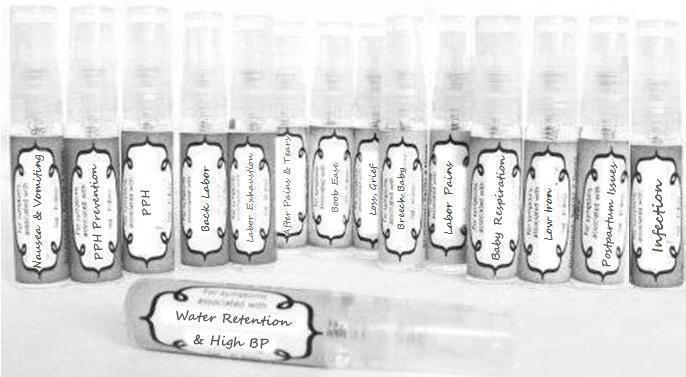
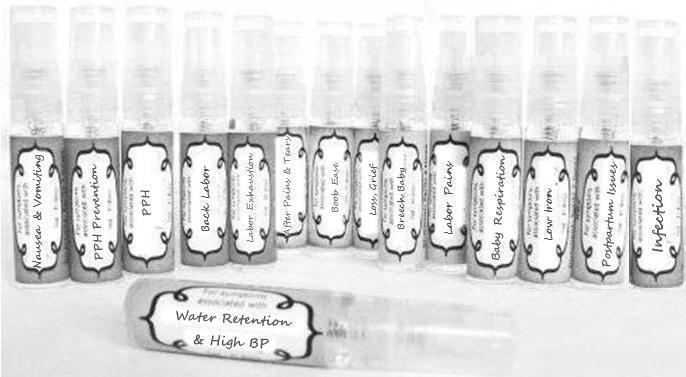
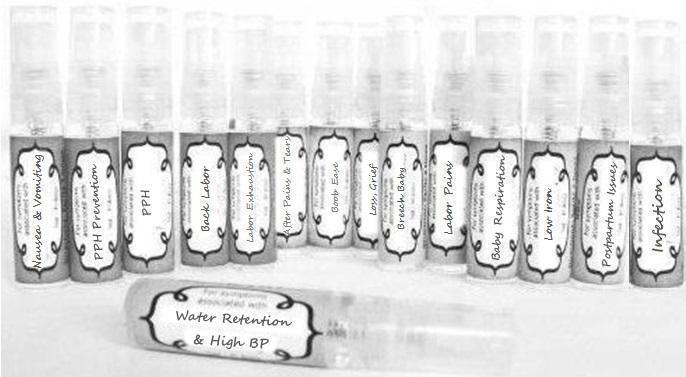
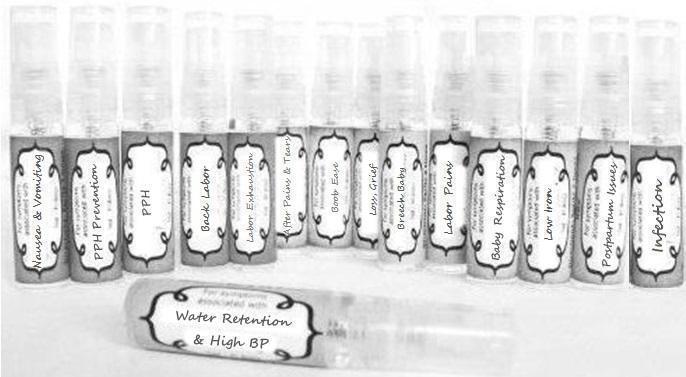
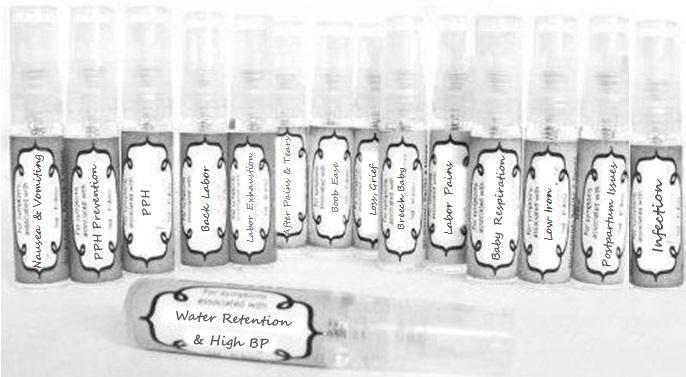
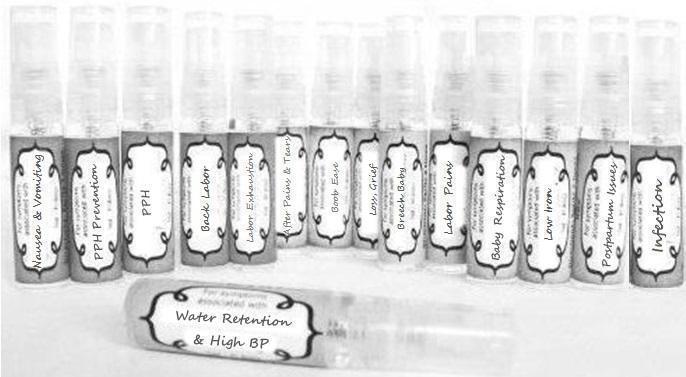
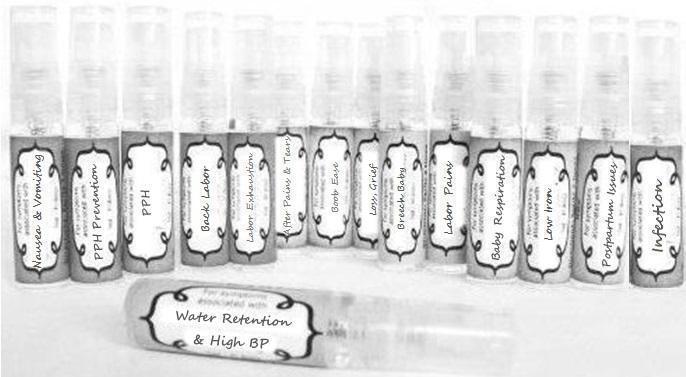
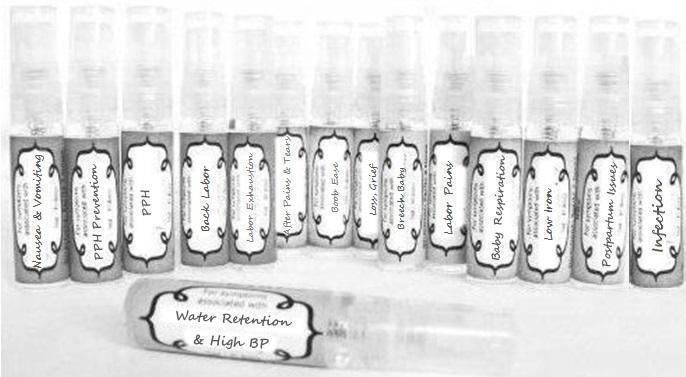
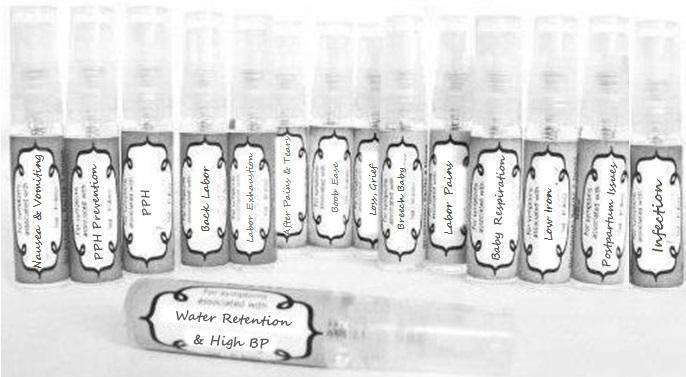
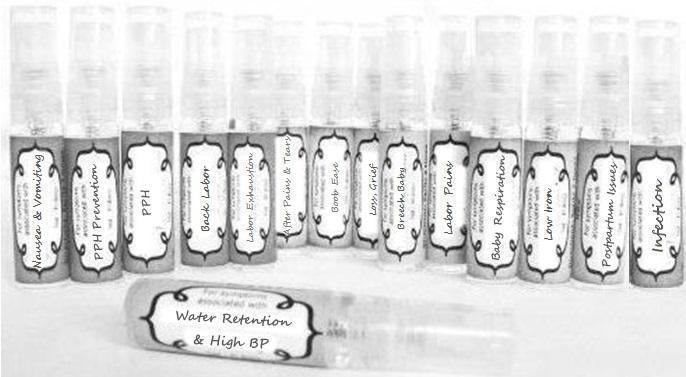
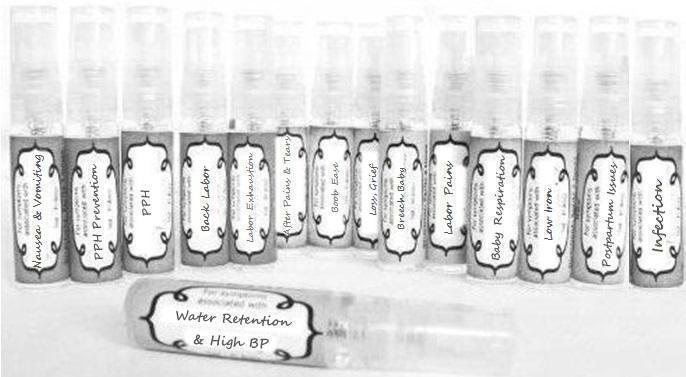
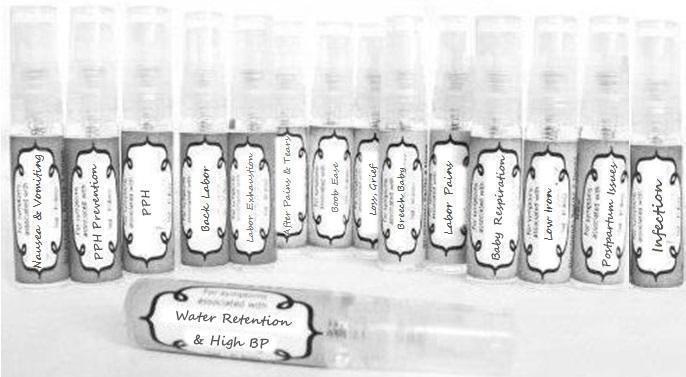
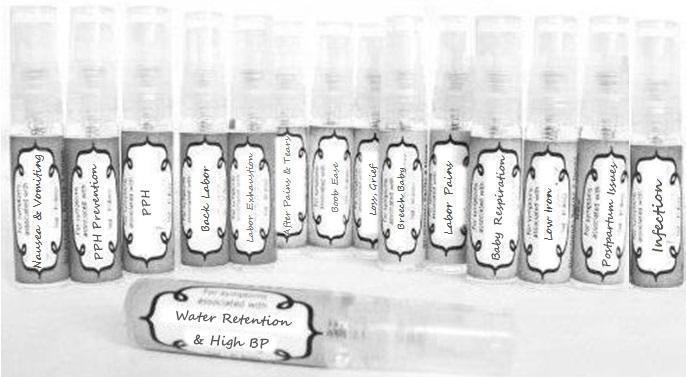
Label: NAUSEA AND VOMITING- aconite 30c, arsenicum album 30c, asarum 30c, conium mac 30c, ipecac 30c, kreosotum 30c, lacticum acidum 30c, medorrhinum 30c, nat mur 30c, nux moschata 30c, nux vomica 30c, phosphorus 30c, pulsatilla 30c, sepia 30c, tobacum 30c, nicotinum 30c spray
WATER RETENTION AND HIGH BP- apis, arsenicum, crotalis hor 30c, digitalis 30c, helonias 30c, kali chlor 30c,, lachesis 30c, lycopodium .......q 30c, chamomilla 30c, croton tig 30c, hamamelis 30c, helonias 30c, ignatia 30c, merc cor 30c, nux vomica 30c, phytolacca 30c, pulsatilla 30c, ratanhia 30c, silicea 30c, sulphur 30c spray
YUMMY MUMMY MILK- aethusa 30c, antimonium crudum 30c, calcarea carb 30c, calcarea phos 30c, chamomilla 30c, ferrum phos 30c, kali bich 30c, natrum carb 30c, phosphoricum acidum 30c, rheum 30c, silicea 30c, valeriana 30c spray
-
Contains inactivated NDC Code(s)
NDC Code(s): 59667-0043-2, 59667-0044-2, 59667-0045-2, 59667-0046-2, view more59667-0047-2, 59667-0048-2, 59667-0049-2, 59667-0050-2, 59667-0051-2, 59667-0052-2, 59667-0053-2, 59667-0054-2, 59667-0055-2, 59667-0056-2, 59667-0057-2, 59667-0058-2, 59667-0059-2 - Packager: Home Sweet Homeopathics
- Category: HUMAN OTC DRUG LABEL
DISCLAIMER: This homeopathic product has not been evaluated by the Food and Drug Administration for safety or efficacy. FDA is not aware of scientific evidence to support homeopathy as effective.
Drug Label Information
Updated August 2, 2013
If you are a healthcare professional or from the pharmaceutical industry please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- PRINCIPAL DISPLAY PANEL
- PURPOSE
- INDICATIONS & USAGE
- Directions:
- KEEP OUT OF REACH OF CHILDREN
- WARNINGS
- Active ingredients:
- Inactive Ingredients:
-
INGREDIENTS AND APPEARANCE
NAUSEA AND VOMITING
aconite 30c, arsenicum album 30c, asarum 30c, conium mac 30c, ipecac 30c, kreosotum 30c, lacticum acidum 30c, medorrhinum 30c, nat mur 30c, nux moschata 30c, nux vomica 30c, phosphorus 30c, pulsatilla 30c, sepia 30c, tobacum 30c, nicotinum 30c sprayProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:59667-0043 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ACONITUM NAPELLUS (UNII: U0NQ8555JD) (ACONITUM NAPELLUS - UNII:U0NQ8555JD) ACONITUM NAPELLUS 30 [hp_C] in 30 [hp_C] ARSENIC ACID (UNII: N7CIZ75ZPN) (ARSENIC ACID - UNII:N7CIZ75ZPN) ARSENIC ACID 30 [hp_C] in 30 [hp_C] ASARUM CANADENSE ROOT (UNII: E2O4O7TQYK) (ASARUM CANADENSE ROOT - UNII:E2O4O7TQYK) ASARUM CANADENSE ROOT 30 [hp_C] in 30 [hp_C] CONIUM MACULATUM FLOWERING TOP (UNII: Q28R5GF371) (CONIUM MACULATUM FLOWERING TOP - UNII:Q28R5GF371) CONIUM MACULATUM FLOWERING TOP 30 [hp_C] in 30 [hp_C] IPECAC (UNII: 62I3C8233L) (IPECAC - UNII:62I3C8233L) IPECAC 30 [hp_C] in 30 [hp_C] WOOD CREOSOTE (UNII: 3JYG22FD73) (WOOD CREOSOTE - UNII:3JYG22FD73) WOOD CREOSOTE 30 [hp_C] in 30 [hp_C] LACTIC ACID, DL- (UNII: 3B8D35Y7S4) (LACTIC ACID, DL- - UNII:3B8D35Y7S4) LACTIC ACID, DL- 30 [hp_C] in 30 [hp_C] GONORRHEAL URETHRAL SECRETION HUMAN (UNII: 9BZG9E3I8F) (GONORRHEAL URETHRAL SECRETION HUMAN - UNII:9BZG9E3I8F) GONORRHEAL URETHRAL SECRETION HUMAN 30 [hp_C] in 30 [hp_C] SODIUM CHLORIDE (UNII: 451W47IQ8X) (CHLORIDE ION - UNII:Q32ZN48698) SODIUM CHLORIDE 30 [hp_C] in 30 [hp_C] NUTMEG (UNII: AEE24M3MQ9) (NUTMEG - UNII:AEE24M3MQ9) NUTMEG 30 [hp_C] in 30 [hp_C] STRYCHNOS NUX-VOMICA SEED (UNII: 269XH13919) (STRYCHNOS NUX-VOMICA SEED - UNII:269XH13919) STRYCHNOS NUX-VOMICA SEED 30 [hp_C] in 30 [hp_C] PHOSPHORUS (UNII: 27YLU75U4W) (PHOSPHORUS - UNII:27YLU75U4W) PHOSPHORUS 30 [hp_C] in 30 [hp_C] PULSATILLA VULGARIS (UNII: I76KB35JEV) (PULSATILLA VULGARIS - UNII:I76KB35JEV) PULSATILLA VULGARIS 30 [hp_C] in 30 [hp_C] SEPIA OFFICINALIS JUICE (UNII: QDL83WN8C2) (SEPIA OFFICINALIS JUICE - UNII:QDL83WN8C2) SEPIA OFFICINALIS JUICE 30 [hp_C] in 30 [hp_C] TOBACCO LEAF (UNII: 6YR2608RSU) (TOBACCO LEAF - UNII:6YR2608RSU) TOBACCO LEAF 30 [hp_C] in 30 [hp_C] NICOTINE (UNII: 6M3C89ZY6R) (NICOTINE - UNII:6M3C89ZY6R) NICOTINE 30 [hp_C] in 30 [hp_C] Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) ALCOHOL (UNII: 3K9958V90M) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:59667-0043-2 30 [hp_C] in 1 BOTTLE, SPRAY 08/02/2013 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved homeopathic 08/02/2013 WATER RETENTION AND HIGH BP
apis, arsenicum, crotalis hor 30c, digitalis 30c, helonias 30c, kali chlor 30c,, lachesis 30c, lycopodium 30c, mercurius corr. 30c, nat mur30c, phosphorus 30c, veratrum vir 30c sprayProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:59667-0044 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength APIS MELLIFERA VENOM (UNII: 76013O881M) (APIS MELLIFERA VENOM - UNII:76013O881M) APIS MELLIFERA VENOM 30 [hp_C] in 30 [hp_C] ARSENIC ACID (UNII: N7CIZ75ZPN) (ARSENIC ACID - UNII:N7CIZ75ZPN) ARSENIC ACID 30 [hp_C] in 30 [hp_C] CROTALUS HORRIDUS HORRIDUS VENOM (UNII: YHA2XLJ956) (CROTALUS HORRIDUS HORRIDUS VENOM - UNII:YHA2XLJ956) CROTALUS HORRIDUS HORRIDUS VENOM 30 [hp_C] in 30 [hp_C] DIGITALIS (UNII: F1T8QT9U8B) (DIGITALIS - UNII:F1T8QT9U8B) DIGITALIS 30 [hp_C] in 30 [hp_C] CHAMAELIRIUM LUTEUM ROOT (UNII: DQV54Y5H3U) (CHAMAELIRIUM LUTEUM ROOT - UNII:DQV54Y5H3U) CHAMAELIRIUM LUTEUM ROOT 30 [hp_C] in 30 [hp_C] POTASSIUM CHLORATE (UNII: H35KS68EE7) (POTASSIUM CATION - UNII:295O53K152) POTASSIUM CHLORATE 30 [hp_C] in 30 [hp_C] LACHESIS MUTA VENOM (UNII: VSW71SS07I) (LACHESIS MUTA VENOM - UNII:VSW71SS07I) LACHESIS MUTA VENOM 30 [hp_C] in 30 [hp_C] LYCOPODIUM CLAVATUM SPORE (UNII: C88X29Y479) (LYCOPODIUM CLAVATUM SPORE - UNII:C88X29Y479) LYCOPODIUM CLAVATUM SPORE 30 [hp_C] in 30 [hp_C] MERCURIC CHLORIDE (UNII: 53GH7MZT1R) (MERCURIC CATION - UNII:ED30FJ8Y42) MERCURIC CHLORIDE 30 [hp_C] in 30 [hp_C] SODIUM CHLORIDE (UNII: 451W47IQ8X) (CHLORIDE ION - UNII:Q32ZN48698) SODIUM CHLORIDE 30 [hp_C] in 30 [hp_C] PHOSPHORUS (UNII: 27YLU75U4W) (PHOSPHORUS - UNII:27YLU75U4W) PHOSPHORUS 30 [hp_C] in 30 [hp_C] VERATRUM VIRIDE ROOT (UNII: 197B3Q7T5Q) (VERATRUM VIRIDE ROOT - UNII:197B3Q7T5Q) VERATRUM VIRIDE ROOT 30 [hp_C] in 30 [hp_C] Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) ALCOHOL (UNII: 3K9958V90M) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:59667-0044-2 30 [hp_C] in 1 BOTTLE, SPRAY Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved homeopathic 08/02/2013 BREECH BABY
ignatia 30c, nat mur 30c, pulsatilla 30c sprayProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:59667-0045 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength STRYCHNOS IGNATII SEED (UNII: 1NM3M2487K) (STRYCHNOS IGNATII SEED - UNII:1NM3M2487K) STRYCHNOS IGNATII SEED 30 [hp_C] in 30 [hp_C] SODIUM CHLORIDE (UNII: 451W47IQ8X) (CHLORIDE ION - UNII:Q32ZN48698) SODIUM CHLORIDE 30 [hp_C] in 30 [hp_C] PULSATILLA VULGARIS (UNII: I76KB35JEV) (PULSATILLA VULGARIS - UNII:I76KB35JEV) PULSATILLA VULGARIS 30 [hp_C] in 30 [hp_C] Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) ALCOHOL (UNII: 3K9958V90M) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:59667-0045-2 30 [hp_C] in 1 BOTTLE, SPRAY Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved homeopathic 08/02/2013 BACK LABOR
cimicifuga 30c, coffea cruda 30c, causticum 30c, kali carb 30c, nux vomica 30c petroleum 30c, sepia 30c sprayProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:59667-0046 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength BLACK COHOSH (UNII: K73E24S6X9) (BLACK COHOSH - UNII:K73E24S6X9) BLACK COHOSH 30 [hp_C] in 30 [hp_C] ARABICA COFFEE BEAN (UNII: 3SW678MX72) (ARABICA COFFEE BEAN - UNII:3SW678MX72) ARABICA COFFEE BEAN 30 [hp_C] in 30 [hp_C] CAUSTICUM (UNII: DD5FO1WKFU) (CAUSTICUM - UNII:DD5FO1WKFU) CAUSTICUM 30 [hp_C] in 30 [hp_C] POTASSIUM CARBONATE (UNII: BQN1B9B9HA) (CARBONATE ION - UNII:7UJQ5OPE7D) POTASSIUM CARBONATE 30 [hp_C] in 30 [hp_C] STRYCHNOS NUX-VOMICA SEED (UNII: 269XH13919) (STRYCHNOS NUX-VOMICA SEED - UNII:269XH13919) STRYCHNOS NUX-VOMICA SEED 30 [hp_C] in 30 [hp_C] PETROLATUM (UNII: 4T6H12BN9U) (PETROLATUM - UNII:4T6H12BN9U) PETROLATUM 30 [hp_C] in 30 [hp_C] SEPIA OFFICINALIS JUICE (UNII: QDL83WN8C2) (SEPIA OFFICINALIS JUICE - UNII:QDL83WN8C2) SEPIA OFFICINALIS JUICE 30 [hp_C] in 30 [hp_C] Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) ALCOHOL (UNII: 3K9958V90M) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:59667-0046-2 30 [hp_C] in 1 BOTTLE, SPRAY Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved homeopathic 08/02/2013 LABOR EXHAUSTION
arnica 30c, belladonna 30c, carbo veg 30c, caulophyllum 30c, cimicifuga 30c, gelsemium 30c, kali carb 30c, kali phos 30c , nat mur 30c, opium 30c, pulsatilla 30c, secale 30c sprayProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:59667-0047 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ARNICA MONTANA (UNII: O80TY208ZW) (ARNICA MONTANA - UNII:O80TY208ZW) ARNICA MONTANA 30 [hp_C] in 30 [hp_C] ATROPA BELLADONNA (UNII: WQZ3G9PF0H) (ATROPA BELLADONNA - UNII:WQZ3G9PF0H) ATROPA BELLADONNA 30 [hp_C] in 30 [hp_C] ACTIVATED CHARCOAL (UNII: 2P3VWU3H10) (ACTIVATED CHARCOAL - UNII:2P3VWU3H10) ACTIVATED CHARCOAL 30 [hp_C] in 30 [hp_C] CAULOPHYLLUM THALICTROIDES ROOT (UNII: JTJ6HH6YEH) (CAULOPHYLLUM THALICTROIDES ROOT - UNII:JTJ6HH6YEH) CAULOPHYLLUM THALICTROIDES ROOT 30 [hp_C] in 30 [hp_C] BLACK COHOSH (UNII: K73E24S6X9) (BLACK COHOSH - UNII:K73E24S6X9) BLACK COHOSH 30 [hp_C] in 30 [hp_C] GELSEMIUM SEMPERVIRENS ROOT (UNII: 639KR60Q1Q) (GELSEMIUM SEMPERVIRENS ROOT - UNII:639KR60Q1Q) GELSEMIUM SEMPERVIRENS ROOT 30 [hp_C] in 30 [hp_C] POTASSIUM CARBONATE (UNII: BQN1B9B9HA) (CARBONATE ION - UNII:7UJQ5OPE7D) POTASSIUM CARBONATE 30 [hp_C] in 30 [hp_C] POTASSIUM PHOSPHATE, DIBASIC (UNII: CI71S98N1Z) (PHOSPHATE ION - UNII:NK08V8K8HR) POTASSIUM PHOSPHATE, DIBASIC 30 [hp_C] in 30 [hp_C] SODIUM CHLORIDE (UNII: 451W47IQ8X) (CHLORIDE ION - UNII:Q32ZN48698) SODIUM CHLORIDE 30 [hp_C] in 30 [hp_C] CLAVICEPS PURPUREA SCLEROTIUM (UNII: 01G9XEA93N) (CLAVICEPS PURPUREA SCLEROTIUM - UNII:01G9XEA93N) CLAVICEPS PURPUREA SCLEROTIUM 30 [hp_C] in 30 [hp_C] PULSATILLA VULGARIS (UNII: I76KB35JEV) (PULSATILLA VULGARIS - UNII:I76KB35JEV) PULSATILLA VULGARIS 30 [hp_C] in 30 [hp_C] Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) ALCOHOL (UNII: 3K9958V90M) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:59667-0047-2 30 [hp_C] in 1 BOTTLE, SPRAY 08/02/2013 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved homeopathic 08/02/2013 PPH PREVENTION
arnica, bellis sprayProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:59667-0048 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ARNICA MONTANA (UNII: O80TY208ZW) (ARNICA MONTANA - UNII:O80TY208ZW) ARNICA MONTANA 30 [hp_C] in 30 [hp_C] BELLIS PERENNIS (UNII: 2HU33I03UY) (BELLIS PERENNIS - UNII:2HU33I03UY) BELLIS PERENNIS 30 [hp_C] in 30 [hp_C] Inactive Ingredients Ingredient Name Strength water (UNII: 059QF0KO0R) ALCOHOL (UNII: 3K9958V90M) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:59667-0048-2 30 [hp_C] in 1 BOTTLE, SPRAY 08/02/2013 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved homeopathic 08/02/2013 PPH
cinchona, cinnamomum sprayProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:59667-0049 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength CINCHONA OFFICINALIS BARK (UNII: S003A158SB) (CINCHONA OFFICINALIS BARK - UNII:S003A158SB) CINCHONA OFFICINALIS BARK 30 [hp_C] in 30 [hp_C] CINNAMON (UNII: 5S29HWU6QB) (CINNAMON - UNII:5S29HWU6QB) CINNAMON 200 [hp_C] in 30 [hp_C] Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) ALCOHOL (UNII: 3K9958V90M) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:59667-0049-2 30 [hp_C] in 1 BOTTLE 08/02/2013 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved homeopathic 08/02/2013 BABY RESPIRATION
aconite, antimonium tart , arnica , carbo veg sprayProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:59667-0050 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ACONITUM NAPELLUS (UNII: U0NQ8555JD) (ACONITUM NAPELLUS - UNII:U0NQ8555JD) ACONITUM NAPELLUS 30 [hp_C] in 30 [hp_C] ANTIMONY POTASSIUM TARTRATE (UNII: DL6OZ476V3) (ANTIMONY CATION (3+) - UNII:069647RPT5) ANTIMONY POTASSIUM TARTRATE 30 [hp_C] in 30 [hp_C] ARNICA MONTANA (UNII: O80TY208ZW) (ARNICA MONTANA - UNII:O80TY208ZW) ARNICA MONTANA 30 [hp_C] in 30 [hp_C] ACTIVATED CHARCOAL (UNII: 2P3VWU3H10) (ACTIVATED CHARCOAL - UNII:2P3VWU3H10) ACTIVATED CHARCOAL 30 [hp_C] in 30 [hp_C] Inactive Ingredients Ingredient Name Strength water (UNII: 059QF0KO0R) ALCOHOL (UNII: 3K9958V90M) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:59667-0050-2 30 [hp_C] in 1 BOTTLE, SPRAY 08/02/2013 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved homeopathic 08/02/2013 AFTER PAINS AND TEARS
arnica 30c, apis 30c, belladonna 30c, calendula 30c, caulophyllum 30c, chamomilla 30c, cimicifuga 30c, ferrum met. 30c, kali carb 30c, sabina 30c, secale 30c, viburnum 30c sprayProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:59667-0051 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ARNICA MONTANA (UNII: O80TY208ZW) (ARNICA MONTANA - UNII:O80TY208ZW) ARNICA MONTANA 30 [hp_C] in 30 [hp_C] APIS MELLIFERA VENOM (UNII: 76013O881M) (APIS MELLIFERA VENOM - UNII:76013O881M) APIS MELLIFERA VENOM 30 [hp_C] in 30 [hp_C] ATROPA BELLADONNA (UNII: WQZ3G9PF0H) (ATROPA BELLADONNA - UNII:WQZ3G9PF0H) ATROPA BELLADONNA 30 [hp_C] in 30 [hp_C] CALENDULA OFFICINALIS FLOWERING TOP (UNII: 18E7415PXQ) (CALENDULA OFFICINALIS FLOWERING TOP - UNII:18E7415PXQ) CALENDULA OFFICINALIS FLOWERING TOP 30 [hp_C] in 30 [hp_C] CAULOPHYLLUM THALICTROIDES ROOT (UNII: JTJ6HH6YEH) (CAULOPHYLLUM THALICTROIDES ROOT - UNII:JTJ6HH6YEH) CAULOPHYLLUM THALICTROIDES ROOT 30 [hp_C] in 30 [hp_C] MATRICARIA RECUTITA (UNII: G0R4UBI2ZZ) (MATRICARIA RECUTITA - UNII:G0R4UBI2ZZ) MATRICARIA RECUTITA 30 [hp_C] in 30 [hp_C] BLACK COHOSH (UNII: K73E24S6X9) (BLACK COHOSH - UNII:K73E24S6X9) BLACK COHOSH 30 [hp_C] in 30 [hp_C] IRON (UNII: E1UOL152H7) (IRON - UNII:E1UOL152H7) IRON 30 [hp_C] in 30 [hp_C] POTASSIUM CARBONATE (UNII: BQN1B9B9HA) (CARBONATE ION - UNII:7UJQ5OPE7D) POTASSIUM CARBONATE 30 [hp_C] in 30 [hp_C] JUNIPERUS SABINA LEAFY TWIG (UNII: Z5BEX9K2G1) (JUNIPERUS SABINA LEAFY TWIG - UNII:Z5BEX9K2G1) JUNIPERUS SABINA LEAFY TWIG 30 [hp_C] in 30 [hp_C] CLAVICEPS PURPUREA SCLEROTIUM (UNII: 01G9XEA93N) (CLAVICEPS PURPUREA SCLEROTIUM - UNII:01G9XEA93N) CLAVICEPS PURPUREA SCLEROTIUM 30 [hp_C] in 30 [hp_C] VIBURNUM OPULUS BARK (UNII: T1UG6H6805) (VIBURNUM OPULUS BARK - UNII:T1UG6H6805) VIBURNUM OPULUS BARK 30 [hp_C] in 30 [hp_C] Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) ALCOHOL (UNII: 3K9958V90M) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:59667-0051-2 30 [hp_C] in 1 BOTTLE, SPRAY 08/02/2013 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved homeopathic 08/02/2013 POSTPARTUM ISSUES
agnus castus 30c, cimicifuga 30c, nat mur 30c, platina 30c, pulsatilla 30c, sepia 30c, sulphur 30c, tarentula 30c veratrum vir 30c sprayProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:59667-0052 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength CHASTE TREE (UNII: 433OSF3U8A) (CHASTE TREE - UNII:433OSF3U8A) CHASTE TREE 30 [hp_C] in 30 [hp_C] BLACK COHOSH (UNII: K73E24S6X9) (BLACK COHOSH - UNII:K73E24S6X9) BLACK COHOSH 30 [hp_C] in 30 [hp_C] SODIUM CHLORIDE (UNII: 451W47IQ8X) (CHLORIDE ION - UNII:Q32ZN48698) SODIUM CHLORIDE 30 [hp_C] in 30 [hp_C] PLATINUM (UNII: 49DFR088MY) (PLATINUM - UNII:49DFR088MY) PLATINUM 30 [hp_C] in 30 [hp_C] PULSATILLA VULGARIS (UNII: I76KB35JEV) (PULSATILLA VULGARIS - UNII:I76KB35JEV) PULSATILLA VULGARIS 30 [hp_C] in 30 [hp_C] SEPIA OFFICINALIS JUICE (UNII: QDL83WN8C2) (SEPIA OFFICINALIS JUICE - UNII:QDL83WN8C2) SEPIA OFFICINALIS JUICE 30 [hp_C] in 30 [hp_C] SULFUR (UNII: 70FD1KFU70) (SULFUR - UNII:70FD1KFU70) SULFUR 30 [hp_C] in 30 [hp_C] LYCOSA TARANTULA (UNII: 86M454L2TT) (LYCOSA TARANTULA - UNII:86M454L2TT) LYCOSA TARANTULA 30 [hp_C] in 30 [hp_C] VERATRUM VIRIDE ROOT (UNII: 197B3Q7T5Q) (VERATRUM VIRIDE ROOT - UNII:197B3Q7T5Q) VERATRUM VIRIDE ROOT 30 [hp_C] in 30 [hp_C] Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) ALCOHOL (UNII: 3K9958V90M) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:59667-0052-2 30 [hp_C] in 1 BOTTLE, SPRAY 08/02/2013 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved homeopathic 08/02/2013 INFECTION
arnica 30c, hepar sulph 30c, kali mur 30c, silicea sprayProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:59667-0053 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ARNICA MONTANA (UNII: O80TY208ZW) (ARNICA MONTANA - UNII:O80TY208ZW) ARNICA MONTANA 30 [hp_C] in 30 [hp_C] CALCIUM SULFIDE (UNII: 1MBW07J51Q) (CALCIUM SULFIDE - UNII:1MBW07J51Q) CALCIUM SULFIDE 30 [hp_C] in 30 [hp_C] POTASSIUM CHLORIDE (UNII: 660YQ98I10) (POTASSIUM CATION - UNII:295O53K152) POTASSIUM CHLORIDE 30 [hp_C] in 30 [hp_C] SILICON DIOXIDE (UNII: ETJ7Z6XBU4) (COLLOIDAL SILICON DIOXIDE - UNII:ETJ7Z6XBU4) SILICON DIOXIDE 30 [hp_C] in 30 [hp_C] Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) ALCOHOL (UNII: 3K9958V90M) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:59667-0053-2 30 [hp_C] in 1 BOTTLE, SPRAY 08/02/2013 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved homeopathic 08/02/2013 LOW IRON
calcarea phos. cinchona, ferrum phos, nat mur sprayProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:59667-0054 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength CINCHONA OFFICINALIS BARK (UNII: S003A158SB) (CINCHONA OFFICINALIS BARK - UNII:S003A158SB) CINCHONA OFFICINALIS BARK 30 [hp_C] in 30 [hp_C] FERROSOFERRIC PHOSPHATE (UNII: 91GQH8I5F7) (FERROSOFERRIC PHOSPHATE - UNII:91GQH8I5F7) FERROSOFERRIC PHOSPHATE 30 [hp_C] in 30 [hp_C] SODIUM CHLORIDE (UNII: 451W47IQ8X) (CHLORIDE ION - UNII:Q32ZN48698) SODIUM CHLORIDE 30 [hp_C] in 30 [hp_C] ANHYDROUS DIBASIC CALCIUM PHOSPHATE (UNII: L11K75P92J) (ANHYDROUS DIBASIC CALCIUM PHOSPHATE - UNII:L11K75P92J) ANHYDROUS DIBASIC CALCIUM PHOSPHATE 30 [hp_C] in 30 [hp_C] Inactive Ingredients Ingredient Name Strength ALCOHOL (UNII: 3K9958V90M) WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:59667-0054-2 30 [hp_C] in 1 BOTTLE, SPRAY 08/02/2013 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved homeopathic 08/02/2013 LOSS, GRIEF AND DEPRESSION
aurum 30c, ignatia 30c, nat mur 30c sprayProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:59667-0055 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength GOLD (UNII: 79Y1949PYO) (GOLD - UNII:79Y1949PYO) GOLD 30 [hp_C] in 30 [hp_C] STRYCHNOS IGNATII SEED (UNII: 1NM3M2487K) (STRYCHNOS IGNATII SEED - UNII:1NM3M2487K) STRYCHNOS IGNATII SEED 30 [hp_C] in 30 [hp_C] SODIUM CHLORIDE (UNII: 451W47IQ8X) (CHLORIDE ION - UNII:Q32ZN48698) SODIUM CHLORIDE 30 [hp_C] in 30 [hp_C] Inactive Ingredients Ingredient Name Strength water (UNII: 059QF0KO0R) 30 [hp_C] in 30 [hp_C] ALCOHOL (UNII: 3K9958V90M) 30 [hp_C] in 30 [hp_C] Product Characteristics Color white Score Shape Size Flavor Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:59667-0055-2 30 [hp_C] in 1 BOTTLE, SPRAY 08/02/2013 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved homeopathic 08/02/2013 BOOB EASE
belladonna 30c, bryonia 30c, calendula 30c, chamomilla 30c, colchicum 30c, graphites 30c, hepar sulph 30c, phytolacca 30c pulsatilla 30c, silicea 30c, urtica urens sprayProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:59667-0056 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ATROPA BELLADONNA (UNII: WQZ3G9PF0H) (ATROPA BELLADONNA - UNII:WQZ3G9PF0H) ATROPA BELLADONNA 30 [hp_C] in 30 [hp_C] BRYONIA ALBA ROOT (UNII: T7J046YI2B) (BRYONIA ALBA ROOT - UNII:T7J046YI2B) BRYONIA ALBA ROOT 30 [hp_C] in 30 [hp_C] CALENDULA OFFICINALIS FLOWERING TOP (UNII: 18E7415PXQ) (CALENDULA OFFICINALIS FLOWERING TOP - UNII:18E7415PXQ) CALENDULA OFFICINALIS FLOWERING TOP 30 [hp_C] in 30 [hp_C] MATRICARIA RECUTITA (UNII: G0R4UBI2ZZ) (MATRICARIA RECUTITA - UNII:G0R4UBI2ZZ) MATRICARIA RECUTITA 30 [hp_C] in 30 [hp_C] COLCHICUM AUTUMNALE SEED (UNII: 17K68Z6JD7) (COLCHICUM AUTUMNALE SEED - UNII:17K68Z6JD7) COLCHICUM AUTUMNALE SEED 30 [hp_C] in 30 [hp_C] GRAPHITE (UNII: 4QQN74LH4O) (GRAPHITE - UNII:4QQN74LH4O) GRAPHITE 30 [hp_C] in 30 [hp_C] CALCIUM SULFIDE (UNII: 1MBW07J51Q) (CALCIUM SULFIDE - UNII:1MBW07J51Q) CALCIUM SULFIDE 30 [hp_C] in 30 [hp_C] PHYTOLACCA AMERICANA ROOT (UNII: 11E6VI8VEG) (PHYTOLACCA AMERICANA ROOT - UNII:11E6VI8VEG) PHYTOLACCA AMERICANA ROOT 30 [hp_C] in 30 [hp_C] PULSATILLA VULGARIS (UNII: I76KB35JEV) (PULSATILLA VULGARIS - UNII:I76KB35JEV) PULSATILLA VULGARIS 30 [hp_C] in 30 [hp_C] SILICON DIOXIDE (UNII: ETJ7Z6XBU4) (COLLOIDAL SILICON DIOXIDE - UNII:ETJ7Z6XBU4) SILICON DIOXIDE 30 [hp_C] in 30 [hp_C] URTICA URENS (UNII: IHN2NQ5OF9) (URTICA URENS - UNII:IHN2NQ5OF9) URTICA URENS 30 [hp_C] in 30 [hp_C] Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) ALCOHOL (UNII: 3K9958V90M) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:59667-0056-2 30 [hp_C] in 1 BOTTLE, SPRAY 08/02/2013 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved homeopathic 08/02/2013 BFING AVERSION
arnica 30c, aurum nat mur 30c, bambusa 30c, calc phos, chamomilla 30c, kali bromatum 30c, lilium tig 30c, nitricum acidum 30c, secale 30c, silicea 30c, sepia 30c, phosphorous 30c, platina 30 sprayProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:59667-0057 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ARNICA MONTANA (UNII: O80TY208ZW) (ARNICA MONTANA - UNII:O80TY208ZW) ARNICA MONTANA 30 [hp_C] in 30 [hp_C] SODIUM TETRACHLOROAURATE (UNII: 7FT6QUT299) (TETRACHLOROAURATE ION - UNII:ZNL6IP5PJX) SODIUM TETRACHLOROAURATE 30 [hp_C] in 30 [hp_C] BAMBUSA BAMBOS WHOLE (UNII: 54FV35D60A) (BAMBUSA BAMBOS WHOLE - UNII:54FV35D60A) BAMBUSA BAMBOS WHOLE 30 [hp_C] in 30 [hp_C] CALCIUM PHOSPHATE, DIBASIC, ANHYDROUS (UNII: L11K75P92J) (ANHYDROUS DIBASIC CALCIUM PHOSPHATE - UNII:L11K75P92J) CALCIUM PHOSPHATE, DIBASIC, ANHYDROUS 30 [hp_C] in 30 [hp_C] MATRICARIA RECUTITA (UNII: G0R4UBI2ZZ) (MATRICARIA RECUTITA - UNII:G0R4UBI2ZZ) MATRICARIA RECUTITA 30 [hp_C] in 30 [hp_C] POTASSIUM BROMIDE (UNII: OSD78555ZM) (BROMIDE ION - UNII:952902IX06) POTASSIUM BROMIDE 30 [hp_C] in 30 [hp_C] LILIUM LANCIFOLIUM WHOLE FLOWERING (UNII: X67Z2963PI) (LILIUM LANCIFOLIUM WHOLE FLOWERING - UNII:X67Z2963PI) LILIUM LANCIFOLIUM WHOLE FLOWERING 30 [hp_C] in 30 [hp_C] NITRIC ACID (UNII: 411VRN1TV4) (NITRIC ACID - UNII:411VRN1TV4) NITRIC ACID 30 [hp_C] in 30 [hp_C] CLAVICEPS PURPUREA SCLEROTIUM (UNII: 01G9XEA93N) (CLAVICEPS PURPUREA SCLEROTIUM - UNII:01G9XEA93N) CLAVICEPS PURPUREA SCLEROTIUM 30 [hp_C] in 30 [hp_C] SEPIA OFFICINALIS JUICE (UNII: QDL83WN8C2) (SEPIA OFFICINALIS JUICE - UNII:QDL83WN8C2) SEPIA OFFICINALIS JUICE 30 [hp_C] in 30 [hp_C] SILICON DIOXIDE (UNII: ETJ7Z6XBU4) (COLLOIDAL SILICON DIOXIDE - UNII:ETJ7Z6XBU4) SILICON DIOXIDE 30 [hp_C] in 30 [hp_C] PHOSPHORUS (UNII: 27YLU75U4W) (PHOSPHORUS - UNII:27YLU75U4W) PHOSPHORUS 30 [hp_C] in 30 [hp_C] PLATINUM (UNII: 49DFR088MY) (PLATINUM - UNII:49DFR088MY) PLATINUM 30 [hp_C] in 30 [hp_C] Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) ALCOHOL (UNII: 3K9958V90M) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:59667-0057-2 30 [hp_C] in 1 BOTTLE, SPRAY 08/02/2013 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved homeopathic 08/02/2013 SORE NIPPLES
arnica 30c, borax 30c calc carb 30c, calendula 30c, castor eq 30c, chamomilla 30c, croton tig 30c, hamamelis 30c, helonias 30c, ignatia 30c, merc cor 30c, nux vomica 30c, phytolacca 30c, pulsatilla 30c, ratanhia 30c, silicea 30c, sulphur 30c sprayProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:59667-0058 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ARNICA MONTANA (UNII: O80TY208ZW) (ARNICA MONTANA - UNII:O80TY208ZW) ARNICA MONTANA 30 [hp_C] in 30 [hp_C] SODIUM BORATE (UNII: 91MBZ8H3QO) (BORATE ION - UNII:44OAE30D22) SODIUM BORATE 30 [hp_C] in 30 [hp_C] OYSTER SHELL CALCIUM CARBONATE, CRUDE (UNII: 2E32821G6I) (OYSTER SHELL CALCIUM CARBONATE, CRUDE - UNII:2E32821G6I) OYSTER SHELL CALCIUM CARBONATE, CRUDE 30 [hp_C] in 30 [hp_C] CALENDULA OFFICINALIS FLOWERING TOP (UNII: 18E7415PXQ) (CALENDULA OFFICINALIS FLOWERING TOP - UNII:18E7415PXQ) CALENDULA OFFICINALIS FLOWERING TOP 30 [hp_C] in 30 [hp_C] CASTOR OIL (UNII: D5340Y2I9G) (CASTOR OIL - UNII:D5340Y2I9G) CASTOR OIL 30 [hp_C] in 30 [hp_C] MATRICARIA RECUTITA (UNII: G0R4UBI2ZZ) (MATRICARIA RECUTITA - UNII:G0R4UBI2ZZ) MATRICARIA RECUTITA 30 [hp_C] in 30 [hp_C] CROTON TIGLIUM SEED (UNII: 0HK2GZK66E) (CROTON TIGLIUM SEED - UNII:0HK2GZK66E) CROTON TIGLIUM SEED 30 [hp_C] in 30 [hp_C] HAMAMELIS VIRGINIANA ROOT BARK/STEM BARK (UNII: T7S323PKJS) (HAMAMELIS VIRGINIANA ROOT BARK/STEM BARK - UNII:T7S323PKJS) HAMAMELIS VIRGINIANA ROOT BARK/STEM BARK 30 [hp_C] in 30 [hp_C] CHAMAELIRIUM LUTEUM ROOT (UNII: DQV54Y5H3U) (CHAMAELIRIUM LUTEUM ROOT - UNII:DQV54Y5H3U) CHAMAELIRIUM LUTEUM ROOT 30 [hp_C] in 30 [hp_C] STRYCHNOS IGNATII SEED (UNII: 1NM3M2487K) (STRYCHNOS IGNATII SEED - UNII:1NM3M2487K) STRYCHNOS IGNATII SEED 30 [hp_C] in 30 [hp_C] MERCURIC CHLORIDE (UNII: 53GH7MZT1R) (MERCURIC CATION - UNII:ED30FJ8Y42) MERCURIC CHLORIDE 30 [hp_C] in 30 [hp_C] STRYCHNOS NUX-VOMICA SEED (UNII: 269XH13919) (STRYCHNOS NUX-VOMICA SEED - UNII:269XH13919) STRYCHNOS NUX-VOMICA SEED 30 [hp_C] in 30 [hp_C] Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) ALCOHOL (UNII: 3K9958V90M) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:59667-0058-2 30 [hp_C] in 1 BOTTLE, SPRAY 08/02/2013 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved homeopathic 08/02/2013 YUMMY MUMMY MILK
aethusa 30c, antimonium crudum 30c, calcarea carb 30c, calcarea phos 30c, chamomilla 30c, ferrum phos 30c, kali bich 30c, natrum carb 30c, phosphoricum acidum 30c, rheum 30c, silicea 30c, valeriana 30c sprayProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:59667-0059 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ARNICA MONTANA (UNII: O80TY208ZW) (ARNICA MONTANA - UNII:O80TY208ZW) ARNICA MONTANA 30 [hp_C] in 30 [hp_C] ATROPA BELLADONNA (UNII: WQZ3G9PF0H) (ATROPA BELLADONNA - UNII:WQZ3G9PF0H) ATROPA BELLADONNA 30 [hp_C] in 30 [hp_C] TRIBASIC CALCIUM PHOSPHATE (UNII: 91D9GV0Z28) (CALCIUM CATION - UNII:2M83C4R6ZB) CALCIUM CATION 30 [hp_C] in 30 [hp_C] MATRICARIA RECUTITA (UNII: G0R4UBI2ZZ) (MATRICARIA RECUTITA - UNII:G0R4UBI2ZZ) MATRICARIA RECUTITA 30 [hp_C] in 30 [hp_C] CALCIUM SULFIDE (UNII: 1MBW07J51Q) (CALCIUM SULFIDE - UNII:1MBW07J51Q) CALCIUM SULFIDE 30 [hp_C] in 30 [hp_C] HYPERICUM PERFORATUM (UNII: XK4IUX8MNB) (HYPERICUM PERFORATUM - UNII:XK4IUX8MNB) HYPERICUM PERFORATUM 30 [hp_C] in 30 [hp_C] MERCURY (UNII: FXS1BY2PGL) (MERCURY - UNII:FXS1BY2PGL) MERCURY 30 [hp_C] in 30 [hp_C] Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) ALCOHOL (UNII: 3K9958V90M) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:59667-0059-2 30 [hp_C] in 1 BOTTLE, SPRAY 08/02/2013 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved homeopathic 08/02/2013 Labeler - Home Sweet Homeopathics (078883277) Establishment Name Address ID/FEI Business Operations Nichole Michelle Langham 078883277 manufacture(59667-0043, 59667-0044, 59667-0045, 59667-0046, 59667-0047, 59667-0048, 59667-0049, 59667-0050, 59667-0051, 59667-0052, 59667-0053, 59667-0054, 59667-0055, 59667-0056, 59667-0057, 59667-0058, 59667-0059) , label(59667-0043, 59667-0044, 59667-0045, 59667-0046, 59667-0047, 59667-0048, 59667-0049, 59667-0050, 59667-0051, 59667-0052, 59667-0053, 59667-0054, 59667-0055, 59667-0056, 59667-0057, 59667-0058, 59667-0059) , pack(59667-0043, 59667-0044, 59667-0045, 59667-0046, 59667-0047, 59667-0048, 59667-0050, 59667-0049, 59667-0051, 59667-0052, 59667-0053, 59667-0054, 59667-0055, 59667-0056, 59667-0057, 59667-0058, 59667-0059)