Label: LORAZEPAM tablet
- NDC Code(s): 68071-5022-5
- Packager: NuCare Pharmaceuticals,Inc.
- This is a repackaged label.
- Source NDC Code(s): 13107-084
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: CIV
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated October 9, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
BOXED WARNING
(What is this?)
WARNING: RISKS FROM CONCOMITANT USE WITH OPIOIDS
Concomitant use of benzodiazepines and opioids may result in profound sedation, respiratory depression, coma, and death (see WARNINGS; PRECAUTIONS, Clinically Significant Drug Interactions).
• Reserve concomitant prescribing of these drugs for use in patients for whom alternative treatment options are inadequate.
• Limit dosages and durations to the minimum required.
• Follow patients for signs and symptoms of respiratory depression and sedation.
Close -
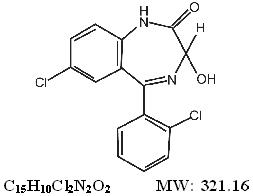
DESCRIPTIONLorazepam, an antianxiety agent, has the chemical formula, 7-chloro-5-( o-chlorophenyl)-1,3-dihydro-3-hydroxy-2 - H-1,4-benzodiazepin-2-one: It is a nearly ...
-
CLINICAL PHARMACOLOGYStudies in healthy volunteers show that in single high doses lorazepam has a tranquilizing action on the central nervous system with no appreciable effect on the respiratory or cardiovascular ...
-
INDICATIONS AND USAGELorazepam tablets USP are indicated for the management of anxiety disorders or for the short-term relief of the symptoms of anxiety or anxiety associated with depressive symptoms. Anxiety or ...
-
CONTRAINDICATIONSLorazepam tablets are contraindicated in patients with - - hypersensitivity to benzodiazepines or to any components of the formulation. - acute narrow-angle glaucoma ...
-
WARNINGSConcomitant use of benzodiazepines, including Lorazepam, and opioids may result in profound sedation, respiratory depression, coma, and death. Because of these risks, reserve concomitant ...
-
PRECAUTIONSIn patients with depression, a possibility for suicide should be borne in mind; benzodiazepines should not be used in such patients without adequate antidepressant therapy ...
-
ADVERSE REACTIONSMost adverse reactions to benzodiazepines, including CNS effects and respiratory depression, are dose dependent, with more severe effects occurring with high doses. In a sample of ...
-
OVERDOSAGEIn postmarketing experience, overdose with lorazepam has occurred predominantly in combination with alcohol and/or other drugs. Therefore, in the management of overdosage, it should be borne in ...
-
DOSAGE AND ADMINISTRATIONLorazepam tablets are administered orally. For optimal results, dose, frequency of administration, and duration of therapy should be individualized according to patient response. To facilitate ...
-
HOW SUPPLIED1 mg, white to off-white, round, flat-faced beveled edge tablets debossed with U33 on one side and bisect on other side. NDC 68071-5022-5 BOTTLES OF 15 - DISPENSE IN A TIGHT ...
-
PACKAGE LABEL-PRINCIPAL DISPLAY PANEL –

-
INGREDIENTS AND APPEARANCEProduct Information