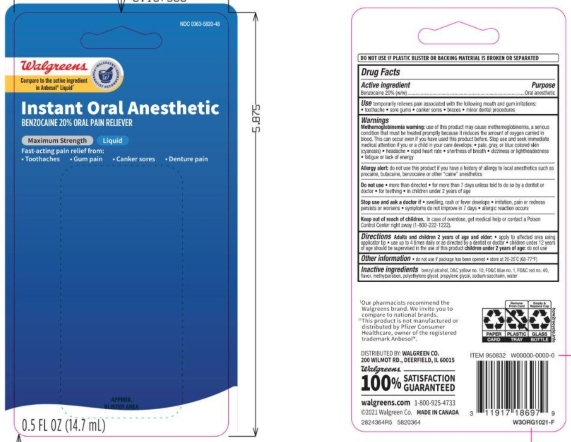
Label: WALGREENS MAXIMUM STRENGTH- benzocaine liquid
- NDC Code(s): 0363-5820-48, 0363-5820-58
- Packager: Walgreens
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated March 27, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- SPL UNCLASSIFIED SECTION
- ACTIVE INGREDIENT
- PURPOSE
- INDICATIONS & USAGE
-
WARNINGS
Warnings
Allergy alert: do not use this product if you have a history of allergy to local anesthetics such as procaine, butacaine, benzocaine or other “caine” anesthetics
When using this product
- avoid contact with eyes
- do not exceed recommended dosage
- do not use more than directed for more than 7 days unless directed by a dentist or doctor.
-
DOSAGE & ADMINISTRATION
Directions
Adults and children 2 years of age and older:
- apply to affect4ed area using applicator tip
- use up to 4 times daily or as directed by a dentist or doctor.
- children under 12 years of age should be supervised in the use of this product.
children under 2 years of age: ask a dentist or doctor.
- STORAGE AND HANDLING
- INACTIVE INGREDIENT
- SPL UNCLASSIFIED SECTION
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
WALGREENS MAXIMUM STRENGTH
benzocaine liquidProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:0363-5820 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength BENZOCAINE (UNII: U3RSY48JW5) (BENZOCAINE - UNII:U3RSY48JW5) BENZOCAINE 20 g in 100 g Inactive Ingredients Ingredient Name Strength BENZYL ALCOHOL (UNII: LKG8494WBH) FD&C RED NO. 40 (UNII: WZB9127XOA) D&C YELLOW NO. 10 (UNII: 35SW5USQ3G) FD&C BLUE NO. 1 (UNII: H3R47K3TBD) WATER (UNII: 059QF0KO0R) POLYETHYLENE GLYCOL, UNSPECIFIED (UNII: 3WJQ0SDW1A) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) SACCHARIN SODIUM (UNII: SB8ZUX40TY) METHYLPARABEN (UNII: A2I8C7HI9T) Product Characteristics Color orange (dark orange/red to sl brown) Score Shape Size Flavor MINT (Mint) Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:0363-5820-58 1 in 1 BLISTER PACK 06/14/2016 1 14.7 g in 1 BOTTLE; Type 1: Convenience Kit of Co-Package 2 NDC:0363-5820-48 1 in 1 BLISTER PACK 06/14/2016 2 14.7 g in 1 BOTTLE; Type 1: Convenience Kit of Co-Package Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M022 01/01/2015 Labeler - Walgreens (008965063) Registrant - Lornamead Inc. (080046418) Establishment Name Address ID/FEI Business Operations HK KOLMAR CANADA, INC 243501959 manufacture(0363-5820)