Label: LOLLICAINE CHERRY- benzocaine gel, dentifrice
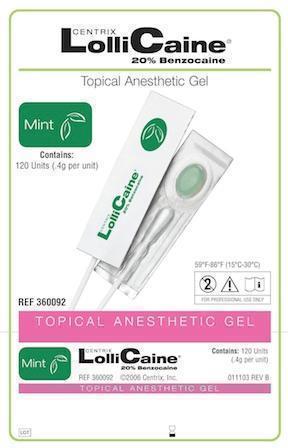
LOLLICAINE MINT- benzocaine gel, dentifrice
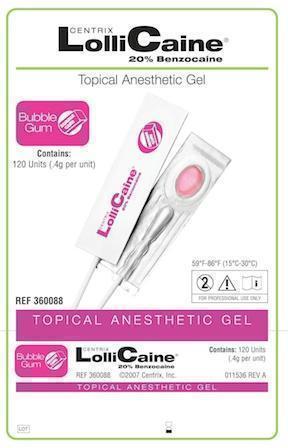
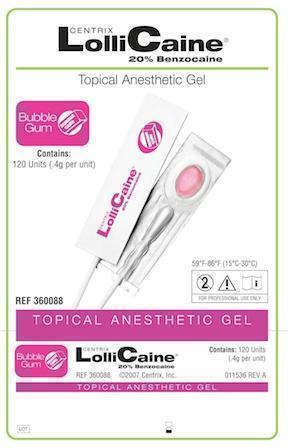
LOLLICAINE BUBBLE GUM- benzocaine gel, dentifrice
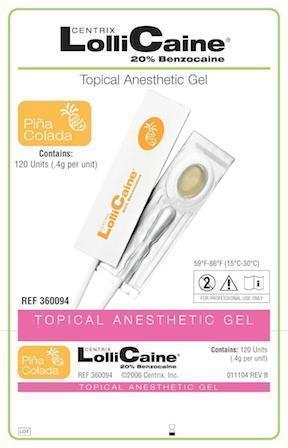
LOLLICAINE PINA COLADA- benzocaine gel, dentifrice
-
Contains inactivated NDC Code(s)
NDC Code(s): 60640-3688-1, 60640-3690-1, 60640-3692-1, 60640-3694-1 - Packager: Centrix, Inc.
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: unapproved drug other
DISCLAIMER: This drug has not been found by FDA to be safe and effective, and this labeling has not been approved by FDA. For further information about unapproved drugs, click here.
Drug Label Information
Updated September 23, 2013
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- HOW SUPPLIED
-
DESCRIPTION
LolliCaine is a flavored topical anesthetic gel featuring rapid onset, no systemic absorption, and 15-minute duration. LolliCaine is packaged in single-dose LolliPacks for maximum asepsis and ease of use. LolliPacks are also ideal to give to patients for take-home, post- operative topical pain relief. Each LolliPackunit contains one unit dose of LolliCaine gel plus swab applicator.
- INDICATIONS & USAGE
- CONTRAINDICATIONS
- WARNINGS
- PRECAUTIONS
-
ADVERSE REACTIONS
All medicines may cause side effects, but many people have no, or minor, side effects. No COMMON side effects have been reported with benzocaine gel. Seek medical attention right away if any of these SEVERE side effects occur:
Severe allergic reactions (rash; hives; itching; difficulty breathing; tightness in the chest; swelling of the mouth, face, lips, or tongue); mouth burning, irritation, redness, swelling, or tenderness.
This is not a complete list of all side effects that may occur. If you have questions about side effects, contact your health care provider.
- INGREDIENTs
- SPL UNCLASSIFIED SECTION
-
SPL UNCLASSIFIED SECTION
SECTION IV: Physical Data: Boiling point: higher than 250 Specific gravity: 1.091/25 Solubility in water: Disperse Viscosity: 164,000 Centipoise (Brookfield, spindle #6, 5 RPM) Appearance and odor: Gel with color and specified flavor Melting point: N/A
pH in water: 6.05
Vapor pressure: Negligible
SECTION V: FIRE AND EXPLOSION DATA: Flash point: Higher than 300 (Non-combustible liquid)
Extinguishing media: Water spray or carbon dioxide
Special fire-fighting procedures: Use self-contained breathing apparatus Unusual fire and explosion hazards: none
Effect of over-exposure: Inhalation of vapors may cause coughing and difficult breathing, contact with skin or eyes may cause irritation. Prolonged skin contact may cause dermati- tis. Ingestion may cause headache, nausea, vomiting, gastrointestinal irritation, convulsions, and unconsciousness
Emergency and first aid procedures: Flush skin and eye contact with water for 15 minutes. If swallowed, induce vomiting at once by giving 2 glasses of water and inserting finger down throat. Call a physician. Remove contaminated clothing and wash. Discard damaged clothing. Chronic effects of over-exposure: not identified.
- SPL UNCLASSIFIED SECTION
- Bubble Gum Package label
- Cherry
- Mint
- Pina Colada
-
INGREDIENTS AND APPEARANCE
LOLLICAINE CHERRY
benzocaine gel, dentifriceProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:60640-3690 Route of Administration DENTAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Benzocaine (UNII: U3RSY48JW5) (Benzocaine - UNII:U3RSY48JW5) Benzocaine 220 mg in 1 g Product Characteristics Color RED Score Shape Size Flavor CHERRY Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:60640-3690-1 120 in 1 BOX, UNIT-DOSE 1 0.4 g in 1 CUP, UNIT-DOSE Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date UNAPPROVED DRUG OTHER 02/19/1963 LOLLICAINE MINT
benzocaine gel, dentifriceProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:60640-3692 Route of Administration DENTAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Benzocaine (UNII: U3RSY48JW5) (Benzocaine - UNII:U3RSY48JW5) Benzocaine 220 mg in 1 g Product Characteristics Color GREEN Score Shape Size Flavor MINT Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:60640-3692-1 120 in 1 BOX, UNIT-DOSE 1 0.4 g in 1 CUP, UNIT-DOSE Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date UNAPPROVED DRUG OTHER 02/19/1963 LOLLICAINE BUBBLE GUM
benzocaine gel, dentifriceProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:60640-3688 Route of Administration DENTAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Benzocaine (UNII: U3RSY48JW5) (Benzocaine - UNII:U3RSY48JW5) Benzocaine 220 mg in 1 g Product Characteristics Color PINK Score Shape Size Flavor BUBBLE GUM Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:60640-3688-1 120 in 1 BOX, UNIT-DOSE 1 0.4 g in 1 CUP, UNIT-DOSE Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date UNAPPROVED DRUG OTHER 02/19/1963 LOLLICAINE PINA COLADA
benzocaine gel, dentifriceProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:60640-3694 Route of Administration DENTAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Benzocaine (UNII: U3RSY48JW5) (Benzocaine - UNII:U3RSY48JW5) Benzocaine 220 mg in 1 g Product Characteristics Color yellow Score Shape Size Flavor COCONUT (Pina Coloda) Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:60640-3694-1 120 in 1 BOX, UNIT-DOSE 1 0.4 g in 1 CUP, UNIT-DOSE Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date UNAPPROVED DRUG OTHER 02/19/1963 Labeler - Centrix, Inc. (053707303) Registrant - Centrix, Inc. (053707303) Establishment Name Address ID/FEI Business Operations Centrix, Inc. 053707303 repack(60640-3690, 60640-3694, 60640-3692, 60640-3688) Establishment Name Address ID/FEI Business Operations DENTSPLY Caulk 083235549 manufacture(60640-3690)