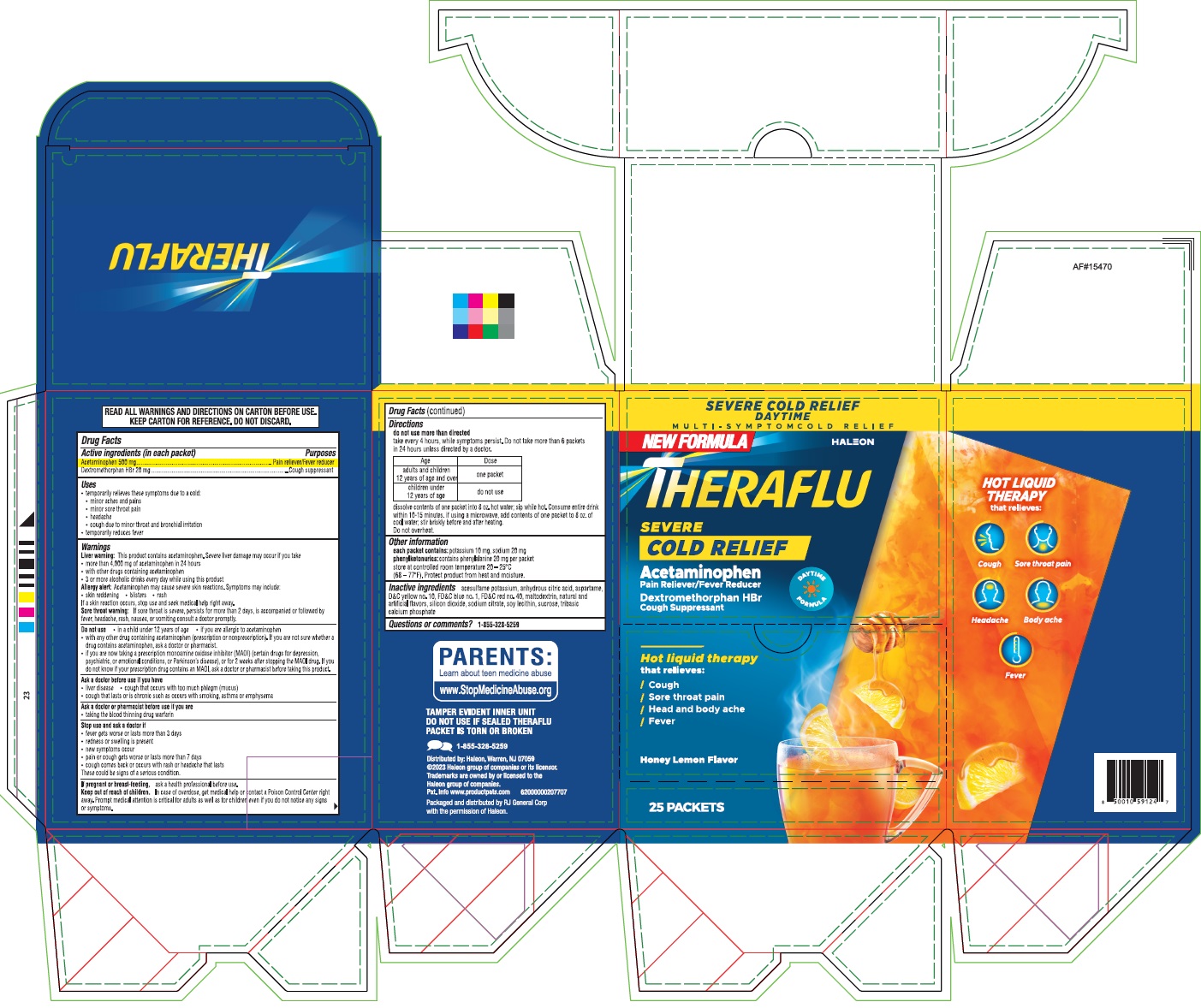
Label: THERAFLU SEVERE COLD RELIEF DAYTIME- acetaminophen, dextromethorphan hbr powder, for solution
- NDC Code(s): 70264-047-01
- Packager: R J General Corporation
- This is a repackaged label.
- Source NDC Code(s): 0067-0100
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated November 15, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
Active ingredients (in each packet)
Active ingredients (in each tablet)Purpose - Acetaminophen 500 mgPain reliever/Fever reducer - Dextromethorphan HBr 20 mgCough Suppressant
- PURPOSE
-
Uses
temporarily relieves these symptoms due to a cold: minor aches and pains - minor sore throat pain - headache - cough due to minor throat and bronchial irritation - temporarily reduces ...
-
Warnings
Liver warning:This product contains acetaminophen. Severe liver damage may occur if you take - more than 4,000 mg of acetaminophen in 24 hours - with other drugs containing acetaminophen - 3 or ...
-
Directions
do not use more than directed - take every 4 hours, while symptoms persist. Do not take more than 6 packets in 24 hours unless directed by a doctor. Age - Dose - adults and children - 12 ...
-
Other information
each packet contains:potassium 10 mg, sodium 20 mg - phenylketonurics:contains phenylalanine 20 mg per packet - store at controlled room temperature 20 - o-25 - oC (68 - o ...
-
Inactive ingredients
acesulfame potassium, anhydrous citric acid, aspartame, D&C yellow no.10, FD&C blue no. 1, FD&C red no. 40, maltodextrin, natural and artificial flavors, silicon dioxide, sodium citrate, soy ...
-
Questions or comments?
1-855-328-5259
-
PRINCIPAL DISPLAY PANELTHERAFLU SEVERE COLD RELIEF - Acetaminophen, Dextromethorphan HBr - NDC 70264-047-01 - 25s Packets Caton Label
-
INGREDIENTS AND APPEARANCEProduct Information