Label: TRIDERMA PRESSURE SORE RELIEF- calendula officinalis cream
- NDC Code(s): 10738-210-41, 10738-210-45, 10738-210-50, 10738-210-55
- Packager: Genuine Virgin Aloe Corporation
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: unapproved homeopathic
DISCLAIMER: This homeopathic product has not been evaluated by the Food and Drug Administration for safety or efficacy. FDA is not aware of scientific evidence to support homeopathy as effective.
Drug Label Information
Updated February 2, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Drug Facts
- Active ingredient
- Purpose
- INDICATIONS & USAGE
-
WARNINGS
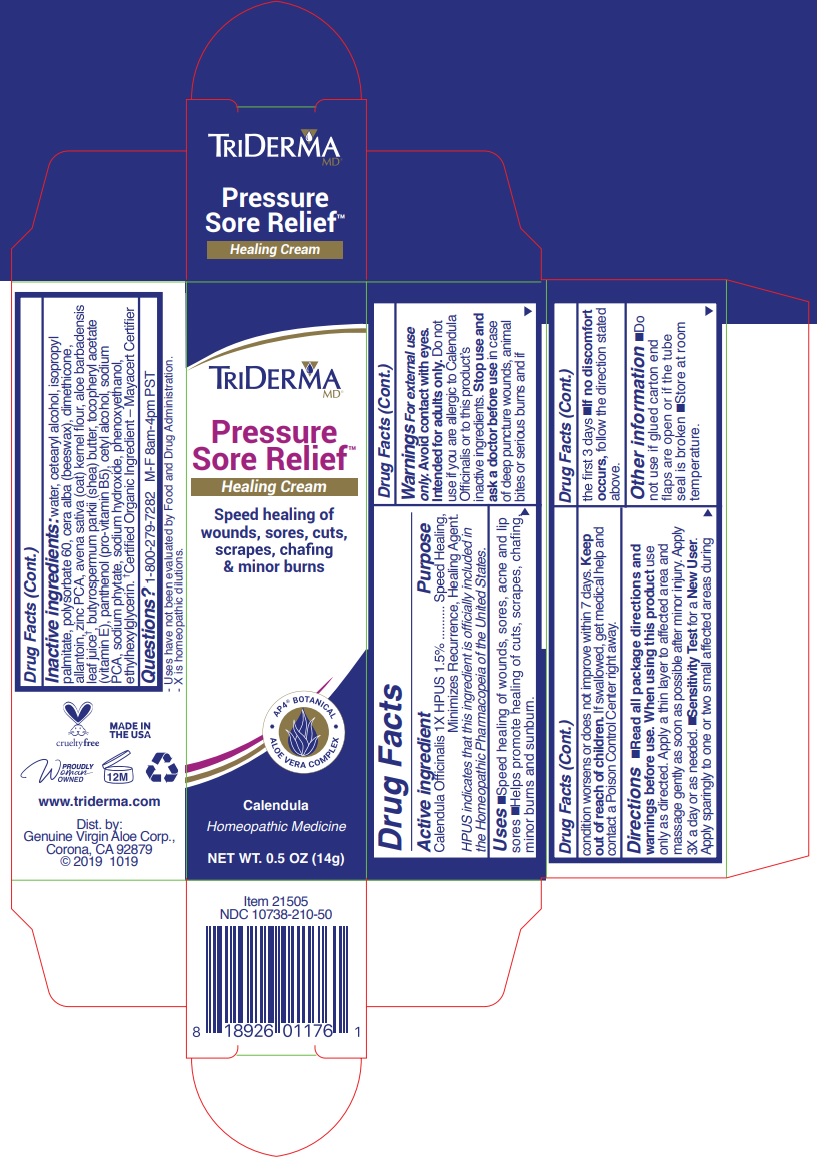
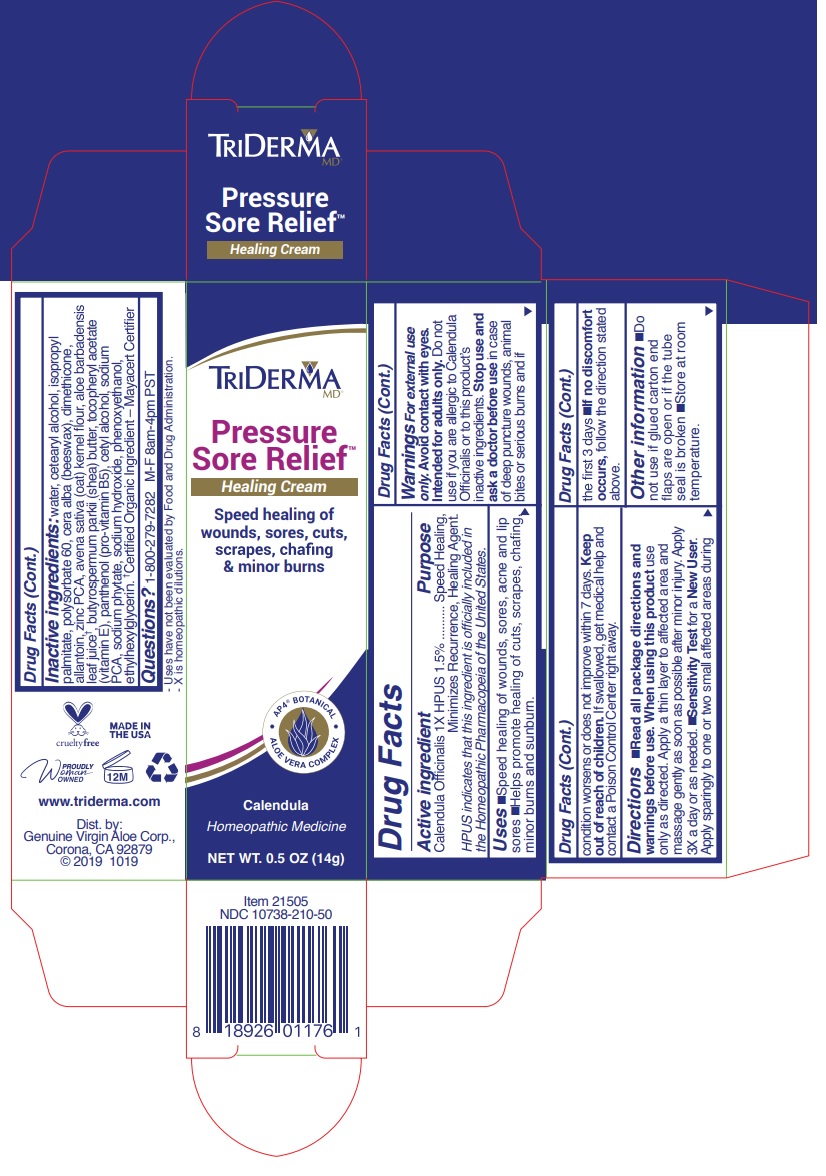
Warnings For external use only.
Avoid contact with eyes. Intended for adults only. Do not use if you are allergic to Calendula Officinalis or to this product's inactive ingredients.
Stop use and ask a doctor before use in case of deep puncture wounds, animal bites or serious burns and if condition persists for more than 4 days or worsens.
-
DOSAGE & ADMINISTRATION
Directions •read all package directions and warnings before use. When using this product use only as directed. Apply a thin layer to affected area and massage gently as soon as possible after minor injury. Apply 3X a day or as needed •Sensitivity Test for a New User. Apply sparingly to one or two small affected areas during the first 3 days. •if no discomfort occurs, follow the direction stated above.
- OTHER SAFETY INFORMATION
-
INACTIVE INGREDIENT
Inactive ingredients: water, cetearyl alcohol, isopropyl palmitate, polysorbate 60, cera alba(beeswax), dimethicone, allantoin, zinc PCA, avena sativa (oat) kernel flour, aloe barbadensis leaf juice †, butyrospermum parkii (shea) butter, tocopheryl acetate (vitamin E), panthenol (pro-vitamin B5), cetyl alcohol, sodium PCA, sodium phytate, sodium hydroxide, phenoxyethanol, ethylhexylglycerin. †Certified Organic Ingredient — Mayacert Certifier
- QUESTIONS
-
SPL UNCLASSIFIED SECTION
Speed healing of wounds, sores, cuts, scrapes, chafing & minor burns
AP4 BOTANICAL
ALOE VERA COMPLEX
Calendula
Homeopathic Medicine- Uses have not been evaluated by Food and Drug Administration.
-X is homeopathic dilutions.
www.triderma.com
Dist. by: Genuine Virgin Aloe corp., Corona, CA 92879 Made in USA
EXPERTS IN SKIN HEALING SINCE 1992
Pressure Sore Relief is specifically formulated to help protect and promote relief for red, irritated, chapped or cracked skin. Use daily to help protect against skin breakdown.
This non-greasy formula has become a customer favorite for those that use a wheelchair, have prosthetic limbs, or are bed-ridden to help keep skin healthy. - Packaging
- Packaging
- Packaging
- Packaging
-
INGREDIENTS AND APPEARANCE
TRIDERMA PRESSURE SORE RELIEF
calendula officinalis creamProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:10738-210 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength CALENDULA OFFICINALIS FLOWERING TOP (UNII: 18E7415PXQ) (CALENDULA OFFICINALIS FLOWERING TOP - UNII:18E7415PXQ) CALENDULA OFFICINALIS FLOWERING TOP 1.5 g in 100 g Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) CETOSTEARYL ALCOHOL (UNII: 2DMT128M1S) ISOPROPYL PALMITATE (UNII: 8CRQ2TH63M) POLYSORBATE 60 (UNII: CAL22UVI4M) YELLOW WAX (UNII: 2ZA36H0S2V) DIMETHICONE (UNII: 92RU3N3Y1O) ALLANTOIN (UNII: 344S277G0Z) ZINC PIDOLATE (UNII: C32PQ86DH4) OATMEAL (UNII: 8PI54V663Y) ALOE VERA LEAF (UNII: ZY81Z83H0X) SHEA BUTTER (UNII: K49155WL9Y) .ALPHA.-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) PANTHENOL (UNII: WV9CM0O67Z) CETYL ALCOHOL (UNII: 936JST6JCN) SODIUM PYRROLIDONE CARBOXYLATE (UNII: 469OTG57A2) PHYTATE SODIUM (UNII: 88496G1ERL) SODIUM HYDROXIDE (UNII: 55X04QC32I) PHENOXYETHANOL (UNII: HIE492ZZ3T) ETHYLHEXYLGLYCERIN (UNII: 147D247K3P) Product Characteristics Color white Score Shape Size Flavor Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:10738-210-50 1 in 1 CARTON 08/30/2019 1 NDC:10738-210-55 14 g in 1 TUBE; Type 0: Not a Combination Product 2 NDC:10738-210-41 113 g in 1 JAR; Type 0: Not a Combination Product 08/30/2019 3 NDC:10738-210-45 113 g in 1 TUBE; Type 0: Not a Combination Product 08/30/2019 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved homeopathic 08/30/2019 Labeler - Genuine Virgin Aloe Corporation (961374147) Establishment Name Address ID/FEI Business Operations Genuine Virgin Aloe Corporation 961374147 manufacture(10738-210)