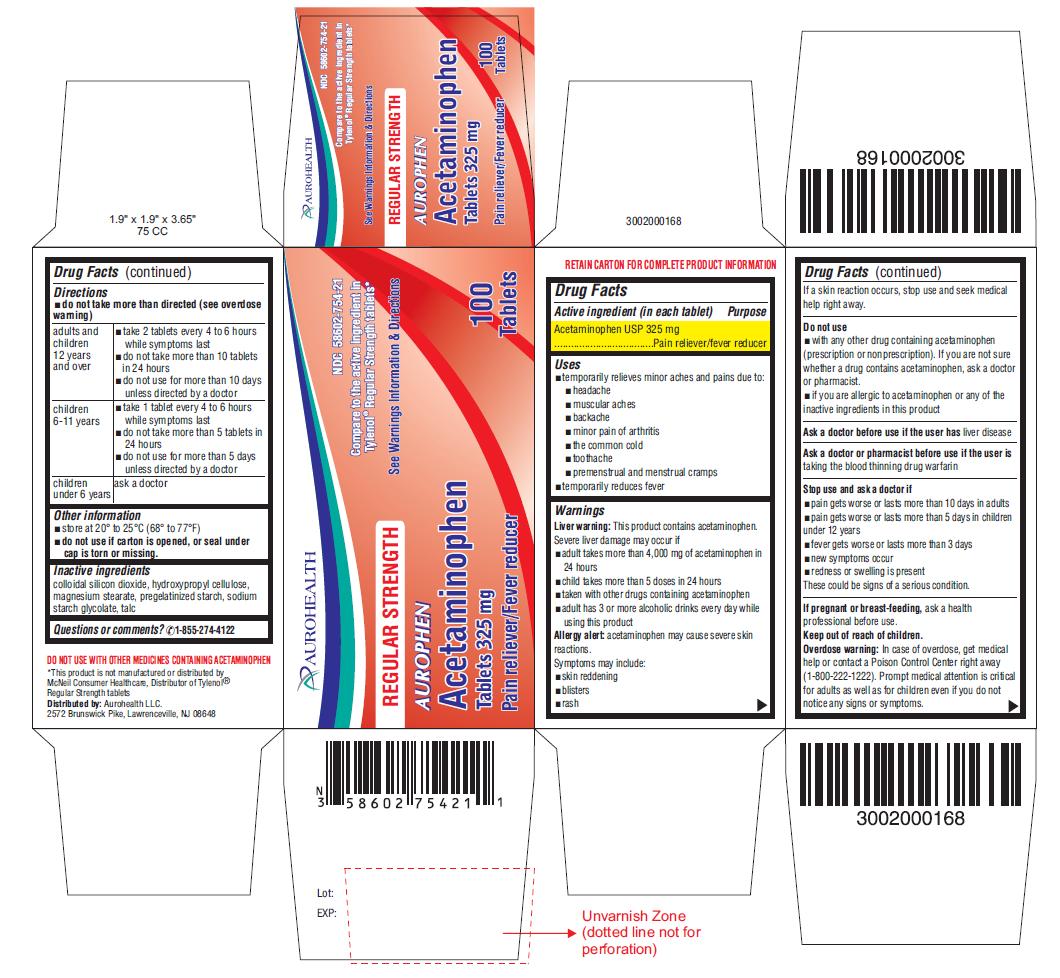
Label: AUROPHEN REGULAR STRENGTH- acetaminophen tablet
- NDC Code(s): 58602-754-07, 58602-754-14, 58602-754-21
- Packager: Aurohealth LLC
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated November 29, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Drug Facts
- Purpose
- Uses
-
Warnings
Liver warning:This product contains acetaminophen.
Severe liver damage may occur if- adult takes more than 4,000 mg of acetaminophen in 24 hours
- child takes more than 5 doses in 24 hours
- taken with other drugs containing acetaminophen
- adult has 3 or more alcoholic drinks every day while using this product
Allergy alert:acetaminophen may cause severe skin reactions.
Symptoms may include:- skin reddening
- blisters
- rash
If a skin reaction occurs, stop use and seek medical help right away.
- Do not use
- Ask a doctor before use if the user has
- Ask a doctor or pharmacist before use if the user is
- Stop use and ask a doctor if
- If pregnant or breast-feeding,
- Keep out of reach of children.
-
Directions
- do not take more than directed (see overdose warning)
adults and children 12 years and over
- take 2 tablets every 4 to 6 hours while symptoms last
- do not take more than 10 tablets in 24 hours
- do not use for more than 10 days unless directed by a doctor
children 6-11 years
- take 1 tablet every 4 to 6 hours while symptoms last
- do not take more than 5 tablets in 24 hours
- do not use for more than 5 days unless directed by a doctor
children under 6 years
ask a doctor
- Other information
- Inactive ingredients
- Questions or comments?
- PACKAGE LABEL-PRINCIPAL DISPLAY PANEL 325 mg (100 Tablets Bottle)
-
INGREDIENTS AND APPEARANCE
AUROPHEN REGULAR STRENGTH
acetaminophen tabletProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:58602-754 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ACETAMINOPHEN (UNII: 362O9ITL9D) (ACETAMINOPHEN - UNII:362O9ITL9D) ACETAMINOPHEN 325 mg Inactive Ingredients Ingredient Name Strength SILICON DIOXIDE (UNII: ETJ7Z6XBU4) HYDROXYPROPYL CELLULOSE (1600000 WAMW) (UNII: RFW2ET671P) MAGNESIUM STEARATE (UNII: 70097M6I30) STARCH, CORN (UNII: O8232NY3SJ) SODIUM STARCH GLYCOLATE TYPE A POTATO (UNII: 5856J3G2A2) TALC (UNII: 7SEV7J4R1U) Product Characteristics Color white (White to off White) Score no score Shape ROUND Size 10mm Flavor Imprint Code N74 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:58602-754-07 1 in 1 CARTON 02/26/2016 1 24 in 1 BOTTLE; Type 0: Not a Combination Product 2 NDC:58602-754-14 1 in 1 CARTON 02/26/2016 2 50 in 1 BOTTLE; Type 0: Not a Combination Product 3 NDC:58602-754-21 1 in 1 CARTON 02/26/2016 3 100 in 1 BOTTLE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M013 02/26/2016 Labeler - Aurohealth LLC (078728447) Establishment Name Address ID/FEI Business Operations APL HEALTHCARE LIMITED 650844777 manufacture(58602-754)