Label: ORENITRAM- treprostinil tablet, extended release
ORENITRAM- treprostinil kit
-
NDC Code(s):
66302-300-01,
66302-300-02,
66302-300-10,
66302-302-01, view more66302-302-02, 66302-302-10, 66302-310-01, 66302-310-02, 66302-310-10, 66302-325-01, 66302-325-02, 66302-325-10, 66302-350-01, 66302-350-02, 66302-350-10, 66302-361-28, 66302-362-56, 66302-363-84
- Packager: United Therapeutics Corporation
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: New Drug Application
Drug Label Information
Updated November 20, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATION
These highlights do not include all the information needed to use ORENITRAM safely and effectively. See Full Prescribing Information for ORENITRAM.
ORENITRAM® (treprostinil) extended-release tablets, for oral use
Initial U.S. Approval: 2002RECENT MAJOR CHANGES
Dosage and Administration (2.1) 08/2023 INDICATIONS AND USAGE
Orenitram is a prostacyclin mimetic indicated for the treatment of pulmonary arterial hypertension (PAH) (WHO Group 1):
- To delay disease progression and to improve exercise capacity. The studies that established effectiveness included predominately patients with WHO functional class II-III symptoms and etiologies of idiopathic or heritable PAH (66%) or PAH associated with connective tissue disease (26%). (1.1)
DOSAGE AND ADMINISTRATION
- Give with food. Swallow tablets whole; use only intact tablets. (2.1)
- Starting dose: 0.125 mg TID or 0.25 mg BID. (2.1)
- Titrate by 0.125 mg TID or by 0.25 mg or 0.5 mg BID, not more frequently than every 3 to 4 days as tolerated. The maximum daily dose is 120 mg. (2.1)
- If transitioning from intravenous (IV) or subcutaneous (SC) Remodulin®, the Orenitram dose should be increased while simultaneously decreasing the IV/SC infusion rate. (2.2)
- Mild hepatic impairment (Child Pugh Class A): Initiate at 0.125 mg BID. Increment at 0.125 mg BID not more frequently than every 3 to 4 days. (2.3)
- Avoid use in patients with moderate hepatic impairment. (2.3)
DOSAGE FORMS AND STRENGTHS
Extended-Release Tablets: 0.125 mg, 0.25 mg, 1 mg, 2.5 mg, and 5 mg. (3)
CONTRAINDICATIONS
- Severe hepatic impairment (Child Pugh Class C). (4)
WARNINGS AND PRECAUTIONS
ADVERSE REACTIONS
Most common adverse reactions (incidence >10%) reported in clinical studies in patients treated with Orenitram compared with placebo are headache, diarrhea, nausea, vomiting, jaw pain, and flushing. (6.1)
To report SUSPECTED ADVERSE REACTIONS, contact United Therapeutics Corp. at 1-866-458-6479 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
DRUG INTERACTIONS
See 17 for PATIENT COUNSELING INFORMATION and FDA-approved patient labeling.
Revised: 8/2023
-
Table of Contents
FULL PRESCRIBING INFORMATION: CONTENTS*
1 INDICATIONS AND USAGE
1.1 Pulmonary Arterial Hypertension
2 DOSAGE AND ADMINISTRATION
2.1 Recommended Dosing
2.2 Transitioning from Subcutaneous or Intravenous Routes of Administration of Treprostinil
2.3 Dose Adjustment in Patients with Hepatic Impairment
2.4 Dose Adjustment for Use with CYP2C8 Inhibitors
2.5 Interruptions and Discontinuation
3 DOSAGE FORMS AND STRENGTHS
4 CONTRAINDICATIONS
5 WARNINGS AND PRECAUTIONS
5.1 Worsening PAH Symptoms upon Abrupt Withdrawal
5.2 Use in Patients with Blind-end Pouches
6 ADVERSE REACTIONS
6.1 Clinical Trials Experience
6.2 Post-Marketing Experience
7 DRUG INTERACTIONS
7.1 Effect of CYP2C8 Inhibitors on Treprostinil
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
8.2 Lactation
8.4 Pediatric Use
8.5 Geriatric Use
8.6 Patients with Hepatic Impairment
8.7 Patients with Renal Impairment
10 OVERDOSAGE
11 DESCRIPTION
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
12.2 Pharmacodynamics
12.3 Pharmacokinetics
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
14 CLINICAL STUDIES
14.1 Clinical Trials in Pulmonary Arterial Hypertension
16 HOW SUPPLIED/STORAGE AND HANDLING
16.1 How Supplied
16.2 Storage
17 PATIENT COUNSELING INFORMATION
- *
- Sections or subsections omitted from the full prescribing information are not listed.
-
1 INDICATIONS AND USAGE
1.1 Pulmonary Arterial Hypertension
Orenitram is indicated for the treatment of pulmonary arterial hypertension (PAH) (WHO Group 1) to delay disease progression and to improve exercise capacity.
The studies that established effectiveness included predominately patients with WHO functional class II-III symptoms and etiologies of idiopathic or heritable PAH (66%) or PAH associated with connective tissue disease (26%).
-
2 DOSAGE AND ADMINISTRATION
2.1 Recommended Dosing
Take Orenitram with food. Swallow Orenitram tablets whole; do not crush, split, or chew.
The recommended starting dose of Orenitram is 0.125 mg three times daily (TID) with food, taken approximately 8 hours apart or 0.25 mg twice daily (BID) with food, taken approximately 12 hours apart.
Titrate by 0.125 mg TID or 0.25 or 0.5 mg BID not more frequently than every 3 to 4 days. Increase the dose to the highest tolerated dose. The recommended maximum daily dose is 120 mg.
If dose increments are not tolerated, consider titrating slower. If intolerable pharmacologic effects occur, decrease the dose in increments of 0.125 mg TID or 0.25 mg BID. Avoid abrupt discontinuation [see Warnings and Precautions (5.1)].
2.2 Transitioning from Subcutaneous or Intravenous Routes of Administration of Treprostinil
Decrease the dose of Remodulin while simultaneously increasing the dose of Orenitram. The dose of Remodulin can be reduced up to 30 ng/kg/min per day and the dose of Orenitram simultaneously increased up to 6 mg per day (2 mg TID) if tolerated. The following equation can be used to estimate a target total daily dose of Orenitram in mg using a patient's dose of intravenous (IV)/subcutaneous (SC) treprostinil (in ng/kg/min) and weight (in kg).
Orenitram total daily dose (mg) = 0.0072 × Remodulin dose (ng/kg/min) × weight (kg)
2.3 Dose Adjustment in Patients with Hepatic Impairment
In patients with mild hepatic impairment (Child Pugh Class A) start at 0.125 mg BID with 0.125 mg BID dose increments not more frequently than every 3 to 4 days. Avoid use of Orenitram in patients with moderate hepatic impairment (Child Pugh Class B). Orenitram is contraindicated in patients with severe hepatic impairment (Child Pugh Class C) due to increases in systemic exposure [see Contraindications (4), Use in Specific Populations (8.6), and Clinical Pharmacology (12.3)].
2.4 Dose Adjustment for Use with CYP2C8 Inhibitors
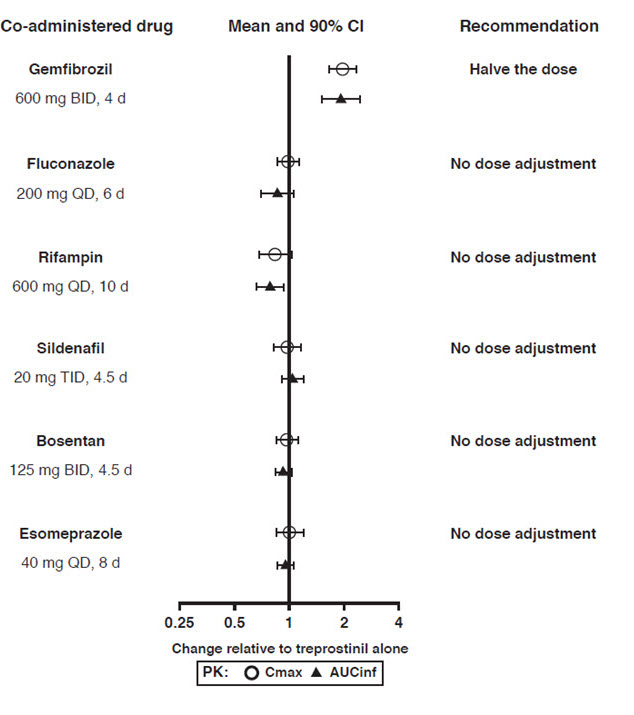
When co-administered with strong CYP2C8 inhibitors (e.g., gemfibrozil) the initial dose is 0.125 mg BID with 0.125 mg BID dose increments not more frequently than every 3 to 4 days.
2.5 Interruptions and Discontinuation
If a dose of medication is missed, the patient should take the missed dose as soon as possible, with food. If a patient misses two or more doses, restart at a lower dose and re-titrate.
In the event of a planned short-term treatment interruption for patients unable to take oral medications, consider a temporary infusion of subcutaneous or intravenous treprostinil. To calculate the total daily dose (mg) of treprostinil for the parenteral route use the following equation:
Remodulin (ng/kg/min) = 139 × Orenitram total daily dose (mg) weight (kg) When discontinuing Orenitram, reduce the dose in steps of 0.5 to 1 mg per day [see Warnings and Precautions (5.1)].
-
3 DOSAGE FORMS AND STRENGTHS
Orenitram (treprostinil) extended-release tablets are available in the following five strengths:
- -
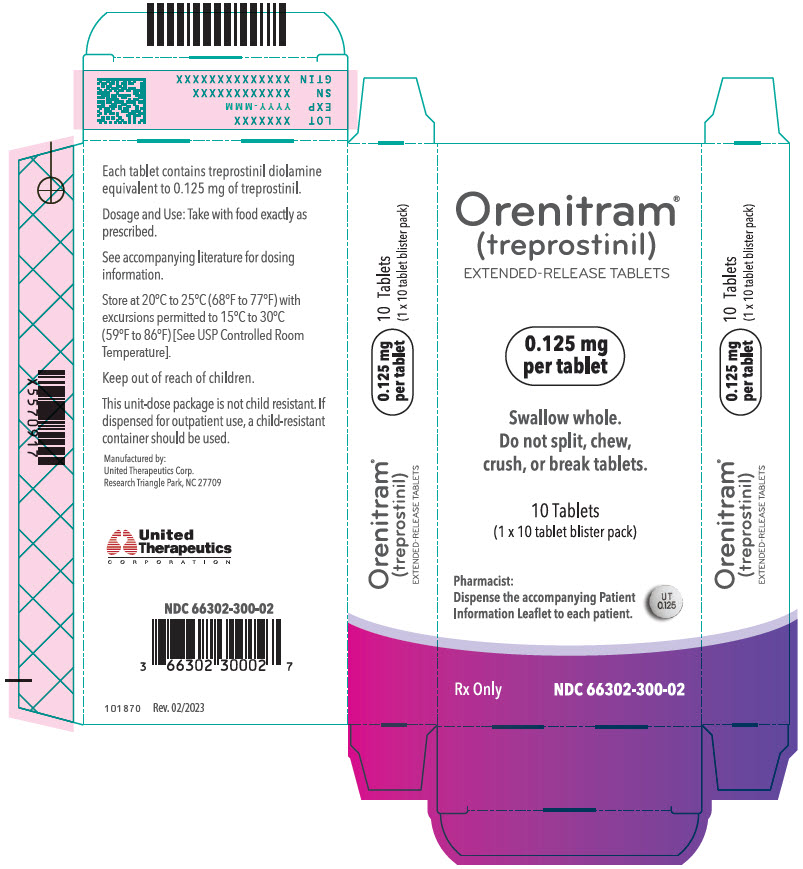
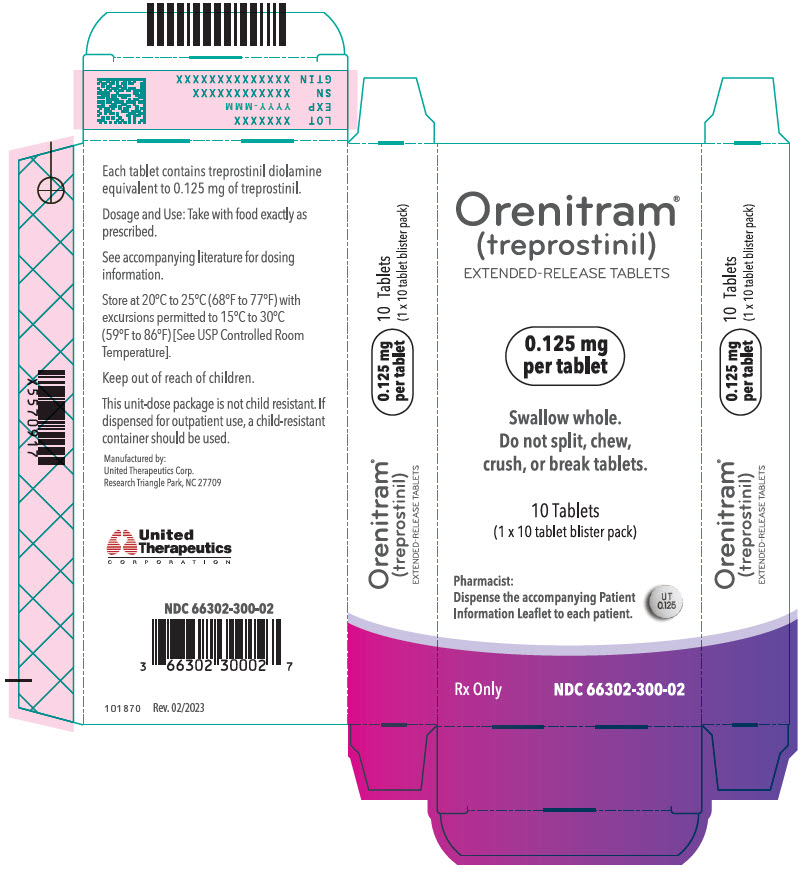
- 0.125 mg [White tablet imprinted with UT 0.125]
- -
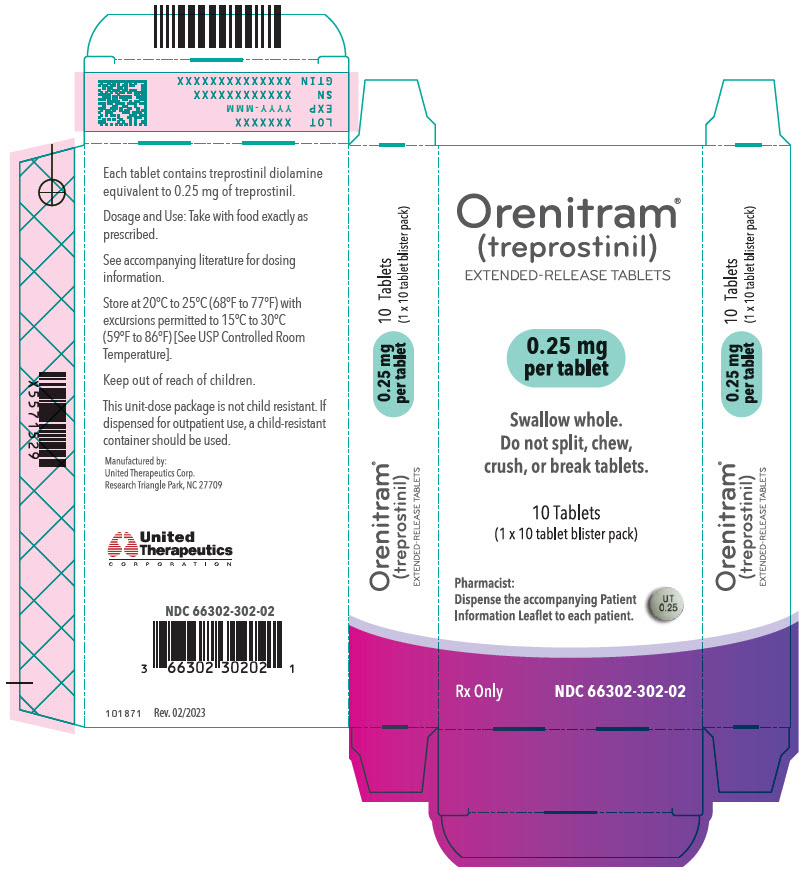
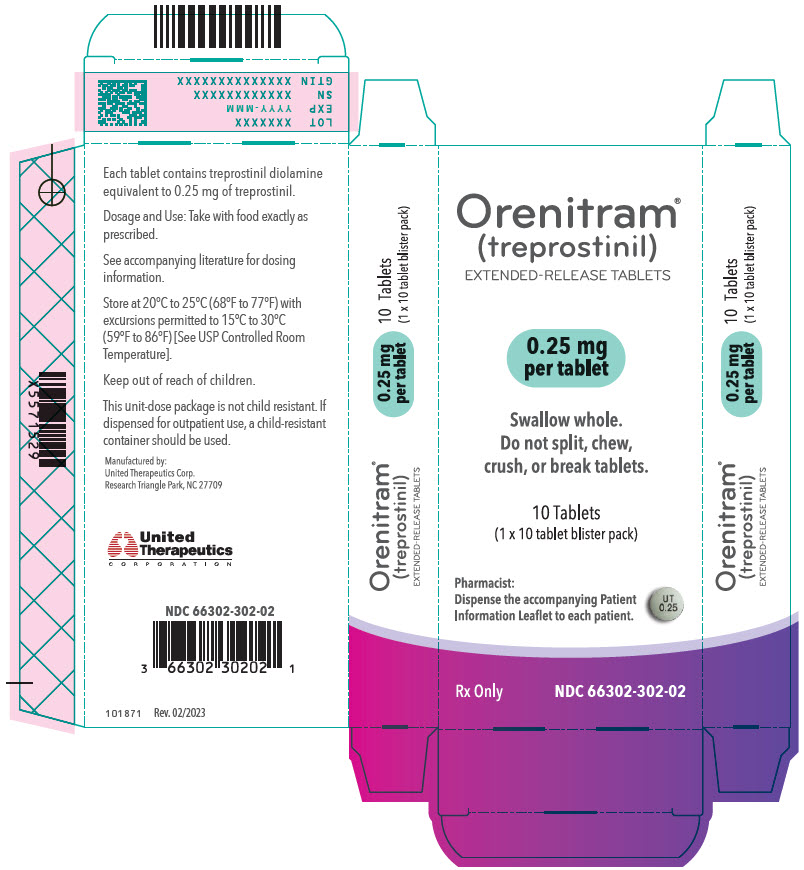
- 0.25 mg [Green tablet imprinted with UT 0.25]
- -
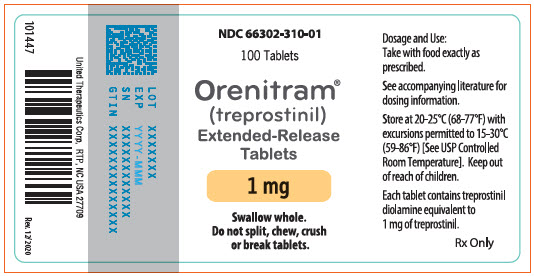
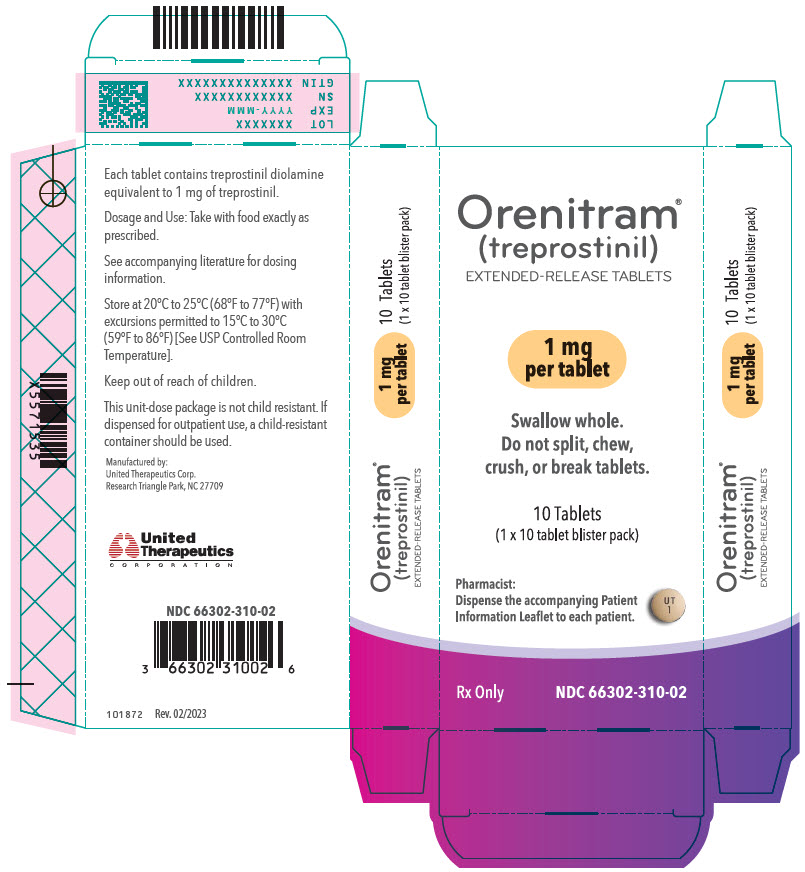
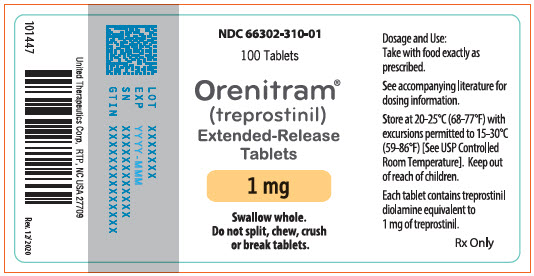
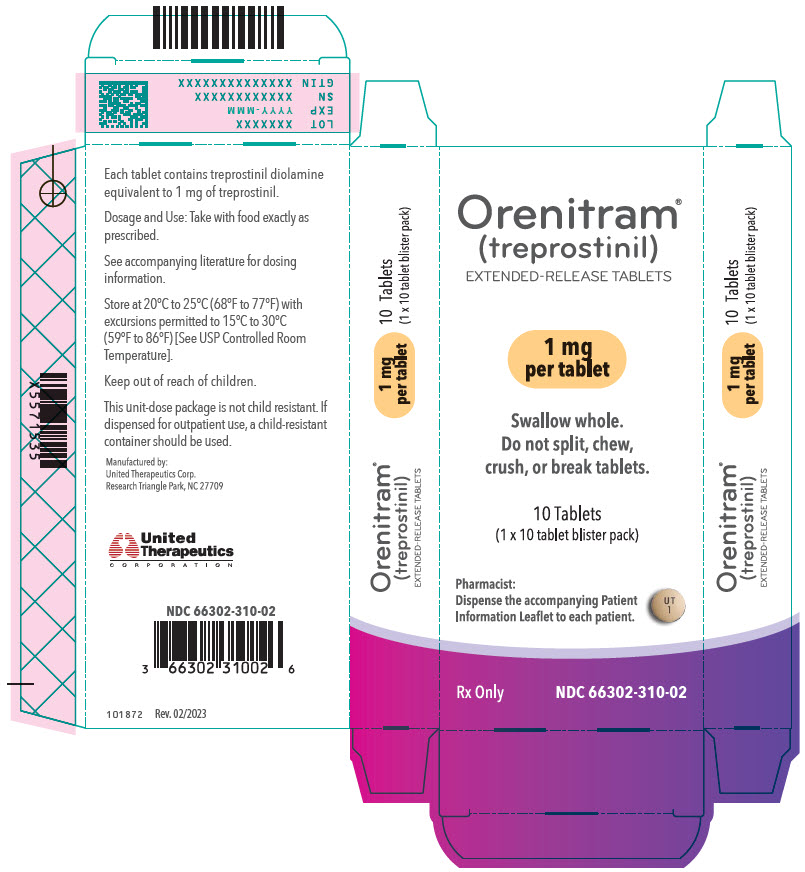
- 1 mg [Yellow tablet imprinted with UT 1]
- -
- 2.5 mg [Pink tablet imprinted with UT 2.5]
- -
- 5 mg [Red tablet imprinted with UT 5]
-
4 CONTRAINDICATIONS
Severe hepatic impairment (Child Pugh Class C) [see Use In Specific Populations (8.6) and Clinical Pharmacology (12.3)].
- 5 WARNINGS AND PRECAUTIONS
-
6 ADVERSE REACTIONS
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in clinical practice.
In a 12-week, placebo-controlled, monotherapy study (Study 1; WHO Group 1; functional class II-III), and an event-driven, placebo-controlled, combination therapy study (Study 4; WHO Group 1; functional class I-III), the most commonly reported adverse reactions that occurred in patients receiving Orenitram included: headache, diarrhea, nausea, and flushing.
Orenitram patients in Study 1 (N=151) had access to 0.25 mg tablets at randomization. Approximately 91% of such patients in Study 1 experienced an adverse reaction, but only 4% discontinued therapy for an adverse reaction (compared to 3% receiving placebo). Study 4 enrolled a total of 690 patients, 346 received Orenitram and 344 received placebo. Overall, 19% of patients treated with Orenitram discontinued treatment in Study 4 due to an adverse event (compared to 4% of patients receiving placebo). The exposure to Orenitram in Study 4 was up to 5.1 years with a median duration of exposure of 1.2 years. Table 1 summarizes adverse events with rates at least 5% higher on Orenitram therapy than on placebo that were reported in either Study 1 or 4.
Table 1: Adverse Events with Rates at Least 5% Higher on Orenitram Therapy than on Placebo in Either Study 1 or Study 4 Reaction Study 1
N=228*Study 4
N=690Orenitram
n=151Placebo
n=77Orenitram
n=346Placebo
n=344- *
- Includes all subjects in the Primary Analysis Population
Headache 63% 19% 75% 35% Diarrhea 30% 16% 69% 29% Nausea 30% 18% 40% 23% Vomiting 17% 16% 36% 10% Flushing 15% 6% 45% 8% Pain in jaw 11% 4% 18% 3% Pain in extremity 14% 8% 18% 9% Hypokalemia 9% 3% 4% 3% Abdominal discomfort 6% 0% 8% 4% Upper abdominal pain 5% 3% 12% 5% Orenitram was studied in a long-term, open-label, extension study in which 824 patients were dosed for a mean duration of approximately 2 years. About 70% of patients continued treatment with Orenitram for at least a year. The mean dose was 4.2 mg BID at one year. The adverse reactions were similar to those observed in the placebo-controlled trials.
The safety of Orenitram was also evaluated in an open-label study transitioning patients from Remodulin. The safety profile during this study was similar to that observed in the three pivotal studies.
6.2 Post-Marketing Experience
The following adverse reactions have been identified during postapproval use of Orenitram: dizziness, dyspepsia, vomiting, myalgia, and arthralgia. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
-
7 DRUG INTERACTIONS
7.1 Effect of CYP2C8 Inhibitors on Treprostinil
Co-administration of Orenitram and the CYP2C8 enzyme inhibitor gemfibrozil in healthy adult volunteers increases exposure to treprostinil. Reduce the starting dose of Orenitram to 0.125 mg BID and use 0.125 mg BID increments not more frequently than every 3 to 4 days [see Dosage and Administration (2.4) and Clinical Pharmacology (12.3)].
-
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Risk Summary
Limited published data from case reports with Orenitram use in pregnant women are not sufficient to assess for a drug-associated risk of major birth defects, miscarriage, or adverse maternal or fetal outcomes. There are risks to the mother and the fetus associated with pulmonary arterial hypertension (see Clinical Considerations). Animal reproductive studies with treprostinil diolamine administered orally have shown an adverse effect on the fetus. In rats, administration of treprostinil to pregnant rats during the period of organogenesis at doses ≥10 mg/kg/day (approximately 15 times the human exposure at the dose of 3.5 mg BID on an AUC basis) resulted in decreased pregnancy rate, increased post-implantation loss, and decreased fetal viability and growth. In rabbits, teratogenicity and decreased fetal viability and growth were observed at doses ≥1.5 mg/kg/day (approximately 7 times the human exposure at the dose of 3.5 mg BID on an AUC basis) (see Animal Data).
The estimated background risk of major birth defects and miscarriage for the indicated populations is unknown. All pregnancies have a background risk of birth defect, loss, or other adverse outcomes. In the U.S. general population, the estimated background risk of major birth defects and miscarriage in clinically recognized pregnancies is 2 to 4% and 15 to 20%, respectively.
Data
In pregnant rats, reversible, dose-dependent decreases in body weight gain and food consumption were observed during the first four days of dosing in animals administered 10, 20, and 30 mg/kg/day treprostinil diolamine. In a dose range-finding study, there was a 17% decrease in the pregnancy rate in the animals administered 20 and 30 mg/kg/day. One dam in each of the 20 and 30 mg/kg/day had litters with no viable fetuses. In the definitive study (0, 5, 10, and 20 mg/kg/day), there were four treatment-related deaths, and a 32% decrease in the pregnancy rate for rats administered 20 mg/kg/day. There was an 8% decrease in the pregnancy rate in the animals administered 10 mg/kg/day. Across both studies, an increase in post-implantation loss was observed in animals administered 10 to 30 mg/kg/day, and a significant decrease in the mean number of live births was seen at dose levels ≥10 mg/kg/day. The no observed adverse effect level was 5 mg/kg/day (maternal, fetal viability and growth), and 20 mg/kg/day (teratogenicity), the highest dose tested in the definitive study. The exposures at 5 and 20 mg/kg/day doses represent 8 and 33 times, respectively, the human exposure at the dose of 3.5 mg BID on an AUC basis.
For F1 progeny, a decreased copulation index was observed at the 5 and 10 mg/kg/day treprostinil diolamine dose levels in rats. The no observed effect levels for physical development, reflex development, exploratory behavior, learning and memory, and sexual maturation was 10 mg/kg/day. The no observed effect level for F1 progeny general development (based on body weight) was 10 mg/kg/day for females and ≤2.5 mg/kg/day for males; the no observed effect level for F1 reproductive performance was 2.5 mg/kg/day (approximately 4 times the human exposure at the dose of 3.5 mg BID on an AUC basis).
In pregnant rabbits, the primary maternal adverse effect was gastrointestinal disturbance; dose-dependent decreases in mean body weight, body weight gain, and food consumption were observed. During the post-dose phase, the effect was reversed. In a dose range-finding study, there was a 17% decrease in the pregnancy rate for animals administered 4 mg/kg/day. A dose-dependent increase in post-implantation loss was observed. Two dams administered 4 mg/kg/day had litters with no viable fetuses; the mean fetal weight was slightly decreased in animals administered 4 mg/kg/day. In the definitive study, mean fetal weights were significantly decreased in animals administered 0.5 to 3 mg/kg/day of treprostinil diolamine. At doses of 1.5 and 3 mg/kg/day, external fetal and soft tissue malformations were observed in a few fetuses, and the total fetal skeletal malformations were significantly increased. The no observed adverse effect level was less than 0.5 mg/kg/day (maternal), 1.5 mg/kg/day (fetal viability and growth), and 0.5 mg/kg/day (teratogenicity). The 0.5 mg/kg/day dose represents about 3 times the human exposure at the dose of 3.5 mg BID on an AUC basis.
8.5 Geriatric Use
Use of Orenitram in patients aged 65 years and over demonstrated slightly higher absolute and relative adverse event rates compared to younger patients. In general, dose selection for an elderly patient should be cautious, reflecting the greater frequency of decreased hepatic or cardiac function, and of concomitant disease or other drug therapy.
8.6 Patients with Hepatic Impairment
There is a marked increase in the systemic exposure to treprostinil in hepatically impaired patients [see Dosage and Administration (2.3), Contraindications (4), and Clinical Pharmacology (12.3)].
8.7 Patients with Renal Impairment
No dose adjustments are required in patients with renal impairment. Orenitram is not removed by dialysis [see Clinical Pharmacology (12.3)].
- 10 OVERDOSAGE
-
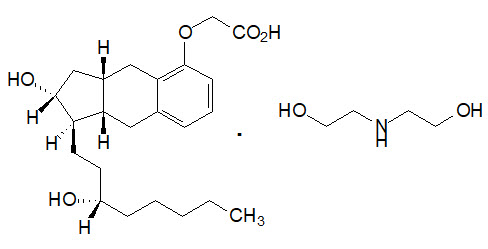
11 DESCRIPTION
Orenitram is an extended-release osmotic tablet for oral administration. Orenitram is formulated as the diolamine salt of treprostinil, a tricyclic benzindene analogue of prostacyclin. The chemical name is Acetic acid, 2-[[(1R,2R,3aS,9aS)-2,3,3a,4,9,9a-hexahydro-2-hydroxy-1-[(3S)-3-hydroxyoctyl]-1H-benz[f]inden-5-yl]oxy]-, complexed with 2,2'-iminobis[ethanol] (1:1). The molecular formula is C23H34O5∙C4H11NO2, the molecular weight is 495.65, and it has the following structural formula:
Orenitram tablets are formulated in five strengths, which contain 0.125 mg of treprostinil (equivalent to 0.159 mg treprostinil diolamine), 0.25 mg of treprostinil (equivalent to 0.317 mg treprostinil diolamine), 1 mg of treprostinil (equivalent to 1.27 mg treprostinil diolamine), 2.5 mg of treprostinil (equivalent to 3.17 mg treprostinil diolamine), or 5 mg of treprostinil (equivalent to 6.35 mg treprostinil diolamine). The formulations also contain xylitol, maltodextrin, sodium lauryl sulfate, magnesium stearate, cellulose acetate, triethyl citrate, polyvinyl alcohol, titanium dioxide, polyethylene glycol, and talc. In addition, tablets may contain colorants FD&C Blue #2, iron oxide yellow, and iron oxide red. The imprint ink contains shellac glaze, ethanol, isopropyl alcohol USP, iron oxide black, n-butyl alcohol, and propylene glycol.
Orenitram is designed to release treprostinil at a near zero-order rate using an osmotic tablet technology. The tablet core is coated with a semi-permeable membrane and has a laser-drilled aperture through the membrane. Upon contact with water (e.g., after ingestion), the core tablet absorbs water through the semi-permeable membrane. The water dissolves the water-soluble treprostinil diolamine and the water-soluble osmotic excipients, which creates hydrostatic pressure within the membrane, eventually forcing the drug across the membrane at a controlled rate.
-
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
The major pharmacologic actions of treprostinil are direct vasodilation of pulmonary and systemic arterial vascular beds, inhibition of platelet aggregation, and inhibition of smooth muscle cell proliferation.
12.2 Pharmacodynamics
In a clinical trial of 240 healthy adult volunteers, single doses of inhaled treprostinil 54 µg (the target clinical dose) and 84 µg (supratherapeutic inhalation dose) prolonged the corrected QTc interval by approximately 10 msec. The QTc effect dissipated rapidly as the concentration of treprostinil decreased. Orenitram has not been evaluated in a thorough QTc study.
12.3 Pharmacokinetics
In patients with PAH, pharmacokinetics of treprostinil is dose-proportional for systemic exposure (AUC0-t) over the dose range from 0.5 to 15 mg BID. Upon repeat administration with a BID regimen, the accumulation in the systemic exposures to treprostinil is minimal and results in a peak-to-trough ratio of approximately 7. However, a TID regimen will reduce the peak-to-trough fluctuations to approximately 2.5 for the same total daily dose.
Absorption
The absolute oral bioavailability of Orenitram is approximately 17%. Maximum treprostinil concentrations occur between approximately 4 and 6 hours following Orenitram administration. Time to reach steady-state concentrations for both BID and TID regimens is approximately 1 to 2 days.
The absorption of Orenitram is affected by food. The AUCinf of treprostinil was increased by 49% and the Cmax was increased by an average of 13% when Orenitram was administered following a high-fat, high-calorie meal compared to fasting conditions in healthy volunteers. The relative bioavailability of treprostinil following oral administration of Orenitram 1 mg is not significantly altered by meal types ranging from 250 to 500 calories in healthy volunteers.
When Orenitram 1 mg was administered with alcohol at 0.5 mg/kg or the equivalent of 3 servings (at the same time, or ± 1 hour relative to alcohol consumption), there was no significant change (10% to 20% increase) in the exposure to treprostinil compared to Orenitram administered alone.
Distribution
The treprostinil component of Orenitram is highly bound to human plasma proteins, approximately 96% over a treprostinil concentration range of 0.01 to 10 µg/mL.
Metabolism and Excretion
In a study conducted in healthy volunteers using [14C] treprostinil, treprostinil was extensively metabolized on the side chain of the molecule via oxidation, oxidative cleavage, dehydration, and glucuronic acid conjugation. Treprostinil is primarily metabolized by CYP2C8 and to a lesser extent by CYP2C9. No new major metabolites are found upon oral administration compared to parenteral administration of treprostinil. Only 1.13% and 0.19% is excreted as unchanged parent drug in the feces and urine, respectively. Based on in vitro studies, treprostinil does not inhibit or induce major CYP enzymes [see Drug Interactions (7.1)].
Specific Populations
Hepatic Impairment: In subjects with mild (n=8) hepatic impairment, administration of a single 1 mg dose of Orenitram resulted in a mean Cmax and an AUC0-inf that were 1.6- and 2.1-fold values seen in healthy subjects, respectively. With moderate impairment (n=8), the corresponding ratios were 4.0- and 4.8-fold, and with severe impairment (n=6), they were 4.8- and 7.6-fold [see Dosage and Administration (2.3), Contraindications (4), and Use in Specific Populations (8.6)].
Drug Interactions
Results of drug interaction studies are shown in Figure 1. Only for the strong CYP2C8 inhibitor does the interaction affect dosing [see Dosage and Administration (2.4)].
Figure 1: Impact of Co-Administered Drugs on the Systemic Exposure of Treprostinil 1 mg Compared to Orenitram Administered Alone
Warfarin: A drug interaction study was carried out with Remodulin co-administered with warfarin (25 mg/day) in healthy volunteers. There was no clinically significant effect of either medication on the pharmacokinetics of treprostinil. Additionally, treprostinil did not affect the pharmacokinetics or pharmacodynamics of warfarin. The pharmacokinetics of R- and S-warfarin and the international normalized ratio (INR) in healthy subjects given a single 25 mg dose of warfarin were unaffected by continuous subcutaneous infusion of treprostinil at an infusion rate of 10 ng/kg/min.
-
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
Treprostinil diolamine did not demonstrate any carcinogenic effects in mouse or rat carcinogenicity studies. Oral administration of treprostinil diolamine to Tg.rasH2 mice at 0, 5, 10, and 20 mg/kg/day in males and 0, 3, 7.5, and 15 mg/kg/day in females daily for 26 weeks did not significantly increase the incidence of tumors. Oral administration of treprostinil diolamine to Sprague Dawley rats at 0, 1, 3, and 10 mg/kg/day daily for 104 weeks did not significantly increase the incidence of tumors. The exposures obtained at the highest dose levels used in males and females are about 13- and 18-fold, respectively, the human exposure at the dose of 3.5 mg BID on an AUC basis.
In vitro genotoxicity studies with high doses of treprostinil did not demonstrate any mutagenic or clastogenic effects. Treprostinil diolamine was tested in vivo in a rat micronucleus assay and did not induce an increased incidence of micronucleated polychromatic erythrocytes.
In rats, treatment with treprostinil diolamine had no effect on reproductive performance or sperm motility at doses up to 10 mg/kg/day. The exposures at this dose level are about 6- (male) to 11- (female) fold the human exposure at the dose of 3.5 mg BID on an AUC basis.
-
14 CLINICAL STUDIES
14.1 Clinical Trials in Pulmonary Arterial Hypertension
Four multicenter, randomized, double-blind studies were conducted and compared Orenitram to placebo in a total of 349 (Study 1), 350 (Study 2), 310 (Study 3), and 690 (Study 4) patients with PAH.
Study 1 (effect seen with no background vasodilator)
Study 1 was a 12-week, randomized (2:1 Orenitram to placebo), double-blind, placebo-controlled, international efficacy and safety study of Orenitram in patients with WHO Group 1 PAH not currently receiving PAH therapy. The primary efficacy endpoint was placebo-corrected change in six-minute walk distance (6MWD) from Baseline to Week 12. Study drug dose was titrated to a maximum of 12 mg BID based on clinical response and study drug tolerability. Study 1 enrolled 349 patients (overall analysis population) who were not receiving any PAH medication. At the beginning of the study, subjects were dosed with only the 1 mg tablets with 0.5 and 0.25 mg tablets introduced at sequentially later dates during the study. The primary analysis population consisted of the 228 patients who had access to the 0.25 mg tablet at the time of randomization. Patients were administered Orenitram or placebo twice daily, with the doses titrated to effect over the course of the 12-week trial. Patients were in WHO functional class II (~33%) and class III (~66%) with either idiopathic or heritable PAH (~75%), collagen vascular disease associated PAH (~19%), or PAH associated with HIV (1%) or congenital heart defect (5%) or other conditions (~6%). The patients' mean baseline 6MWD was approximately 330 meters. In the primary analysis population, 17% of patients discontinued Orenitram compared to 14% of patients on placebo.
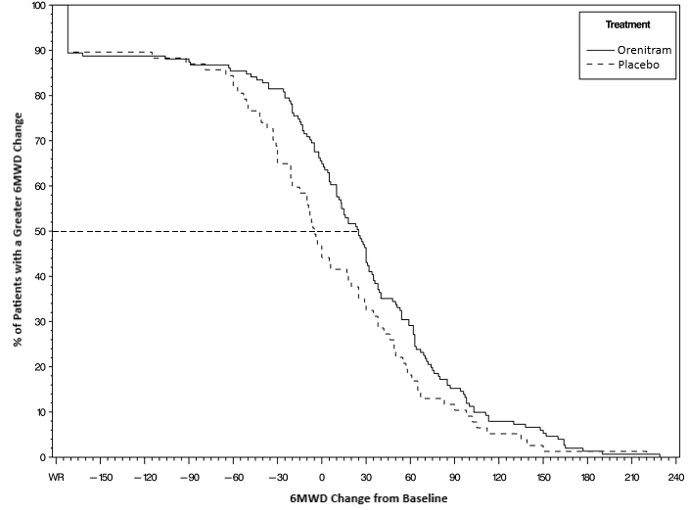
The primary efficacy endpoint of the trial was the change in 6MWD at 12 weeks for the primary analysis population. Analysis of Study 1 results demonstrated that those patients receiving Orenitram compared to patients receiving placebo improved their median change in 6MWD by approximately 23 meters (p=0.013) as compared to patients receiving placebo as demonstrated in (Figure 2). The within group median change from baseline was +25 meters for Orenitram and -5 meters for placebo at Week 12 (N=228). Mean dose (±SD) in the Orenitram group was 2.3 ± 1.3, 3.2 ± 1.9, and 3.4 ± 1.9 mg BID at Weeks 4, 8, and 12, respectively, with a maximum dose of 12 mg BID. The distribution of the 6MWD change from baseline at Week 12 was also plotted across the range of observed values (Figure 3).
Figure 2: Estimate of Treatment Effect by Visit for the Primary Analysis Population (Study 1)
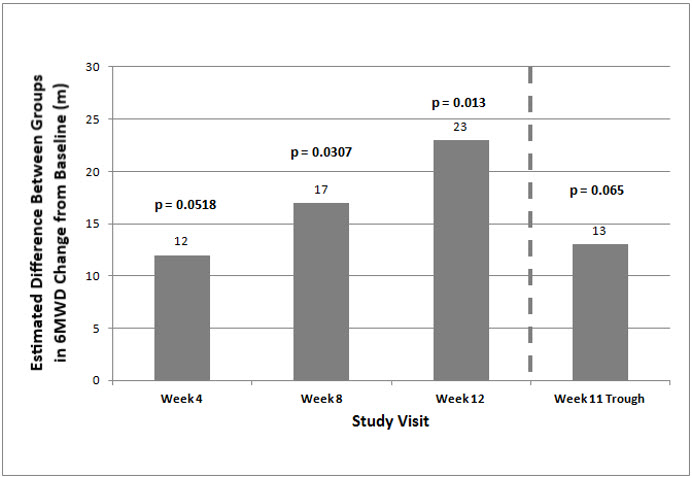
Figure 3: Plot of the Distribution of Peak 6MWD Changes at Week 12 for the Primary Analysis Population (Study 1)
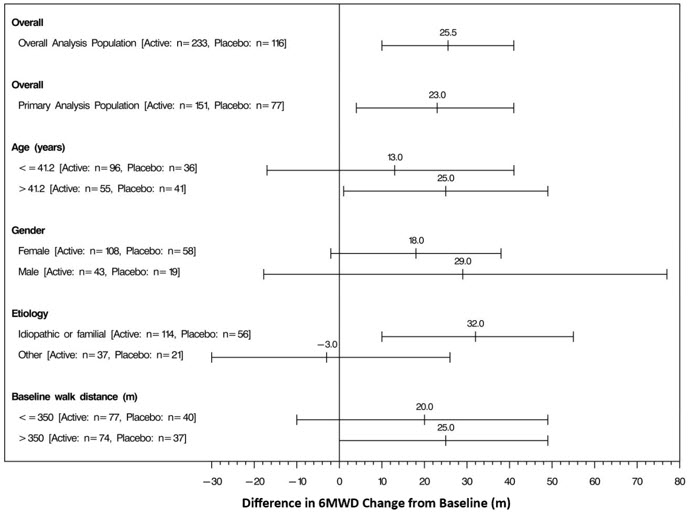
The placebo-corrected median treatment effect on 6MWD was estimated within various subpopulations defined by age, gender, disease etiology, and baseline 6MWD (Figure 4).
Figure 4: Placebo Corrected Median Treatment Effect (with 95% CI) on 6MWD Change from Baseline at Week 12 for Various Subgroups in the Primary Analysis Population (Study 1)
Studies 2 and 3 (no effect on a background of ERA, PDE-5 inhibitor, or both)
Studies 2 (N=350) and 3 (N=310) were 16-week, randomized, double-blind, placebo-controlled, international efficacy and safety studies of Orenitram in patients with WHO Group 1 PAH. The primary efficacy endpoint was placebo-corrected change in 6MWD from Baseline to Week 16. Patients were in WHO functional class II (~23%) and class III (~77%) with either idiopathic or heritable PAH (~66%), collagen vascular disease associated PAH (~29%), or PAH associated with HIV (1%) or congenital heart defect (4%). The patients' mean baseline 6MWD was approximately 340 meters. Approximately 40% were receiving both an ERA and a PDE-5 inhibitor. The results did not demonstrate a benefit in exercise testing with median 6MWD at Week 16.
Study 4 (effect seen with a single background PAH therapy)
The effect of Orenitram on the progression of PAH was demonstrated in an international, multicenter, double-blind, event-driven study in patients with WHO Group 1 PAH randomized 1:1 to Orenitram or placebo (Study 4). The primary efficacy endpoint was the time to first clinical worsening (morbidity or mortality) event. Study drug dose was titrated starting at 0.125 mg TID to a maximum of 12 mg TID based on clinical response and study drug tolerability. Study 4 enrolled a total of 690 patients (primary efficacy analysis population) who were currently receiving a single approved PAH therapy (PDE-5 inhibitor or soluble guanylate cyclase (sGC) [72%]; ERA [28%]). The median age was 43 years and most patients were white (52%) and female (79%). The majority of patients were lower risk in WHO functional class II (63%) with a mean (±SD) baseline 6MWD of 396 (±96) meters. Most patients had either idiopathic or heritable PAH (63%) or collagen vascular disease associated PAH (26%).
Patients treated with Orenitram achieved a median dose of 3.6 mg TID at Week 24, which continued to increase until approximately Week 60 where the median dose of Orenitram was approximately 5 mg TID.
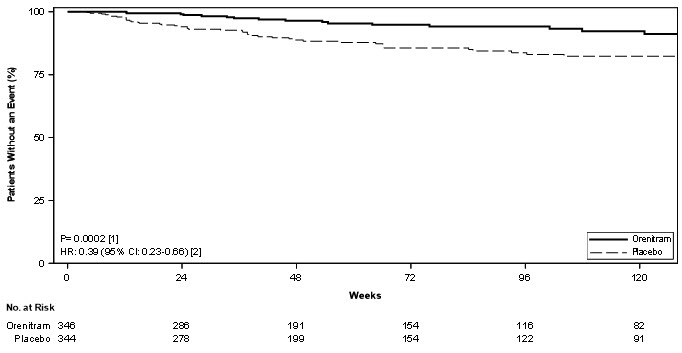
Treatment with Orenitram resulted in a significant increase in the time to first clinical worsening event compared with patients who received placebo, which was associated with a reduction in the risk of an event (HR=0.75 [95% CI; 0.57, 0.99]; p=0.039; Figure 5). The beneficial effect of Orenitram was primarily attributable to a delay in disease progression—defined as a 15% decline in 6MWD plus an increase in either WHO Functional Class or worsening of signs or symptoms of right heart failure—(HR=0.39 [95% CI; 0.23, 0.66]; Figure 6), but there was no effect on the other components of clinical worsening (Table 2).
Figure 5: Kaplan-Meier Plot of Time to Clinical Worsening Events for the Primary Analysis Population (Study 4)
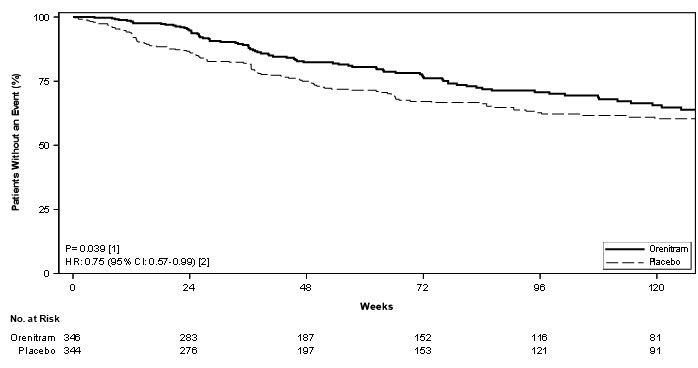
Table 2: Primary Endpoint Events (Study 4) Orenitram
% N=346Placebo
% N=344HR (95% CI) 6MWD, 6-minute walk distance; CI, confidence interval; HR, hazard ratio; IV PGI2, intravenously administered prostacyclin; PAH, pulmonary arterial hypertension; RHF, right heart failure; WHO, World Health Organization Clinical worsening 26 36 0.75 (0.57, 0.99) Any of these All-cause mortality 4.3 4.1 Hospitalization for PAH 10 10 Inhaled/IV PGI2 0.6 1.5 Unsatisfactory long-term clinical response 5.5 5.8 Disease progression 5.5 14.5 0.39 (0.23, 0.66) This → 15% decrease in 6MWD 5.5 14.5 Plus one of these Increase in WHO functional class 2.6 8.1 Increased RHF symptoms 2.9 6.4 Figure 6: Kaplan-Meier Plot of Time to Disease Progression for the Primary Analysis Population (Study 4)
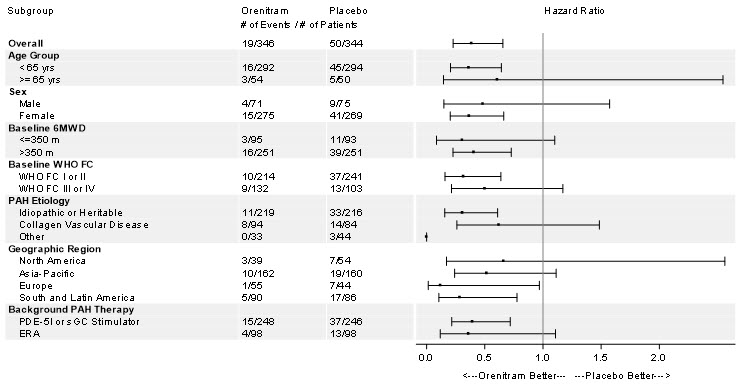
The treatment effect on time to first clinical worsening event due to disease progression was consistent for various subgroups defined by age, sex, baseline 6MWD, WHO functional class, disease etiology, geographical regions, and background therapy (Figure 7).
Figure 7: Forest Plot of Subgroup Analyses of Time to Disease Progression for Various Subgroups in the Primary Analysis Population (Study 4)
Long-Term Treatment of Pulmonary Arterial Hypertension
Patients (N=824) from the placebo-controlled studies entered a long-term, uncontrolled, open-label extension study. The average exposure to Orenitram was approximately 2 years with a maximum exposure of approximately 6 years. The dose of Orenitram continued to increase over time with doses (mean ± SD) of 3.6 ± 2.7, 4.2 ± 3.1, and 5 ± 3.7 mg BID at 6 (n=649), 12 (n=433), and 24 months (n=238), respectively, with a maximum dose of 21 mg BID. Reasons for discontinuation from the study included adverse event (16%), progression of disease (15%), death (13%), and withdrawn consent (7%). In the 522 subjects that completed the 12-month efficacy assessment, their mean 6MWD improved by 24 meters compared to baseline (30 meters in monotherapy patients and 20 meters when Orenitram was used in combination with an ERA and/or a PDE-5 inhibitor). Of the patients that remained in the study, overall survival was 92%, 87%, and 82% at the end of 1, 2, and 3 years, respectively, with progression-free survival (progression defined as death, discontinuation, or addition of a PAH therapy) of 74%, 61%, and 47%. Without a control group, these data must be interpreted cautiously.
Remodulin to Orenitram Transition Study
A 24-week, multicenter, open-label study enrolled 33 WHO Group 1 patients on stable doses of Remodulin. All patients received background therapy with a PDE-5 inhibitor and/or ERA. Patients were WHO functional class I or II and hemodynamically stable at baseline with a cardiac index >2.2 L/m2, RAP <11 mmHg, and PVR <10 Wood units. The primary endpoint of the study was the safety and tolerability of the transition. Successful transition was defined as transition from Remodulin to Orenitram at Week 4 (no longer receiving Remodulin) and clinically maintained on Orenitram through Week 24 (as measured by 6MWD and hemodynamics).
All patients transitioned from Remodulin to Orenitram (median time to transition of 3 days) with thirty-one patients (94%) completing transition in 5 days (range 2 to 29 days). Two subjects discontinued Orenitram. After 24 weeks of treatment with Orenitram, 6MWD and hemodynamics remained stable. Without a control group, these data must be interpreted cautiously.
-
16 HOW SUPPLIED/STORAGE AND HANDLING
16.1 How Supplied
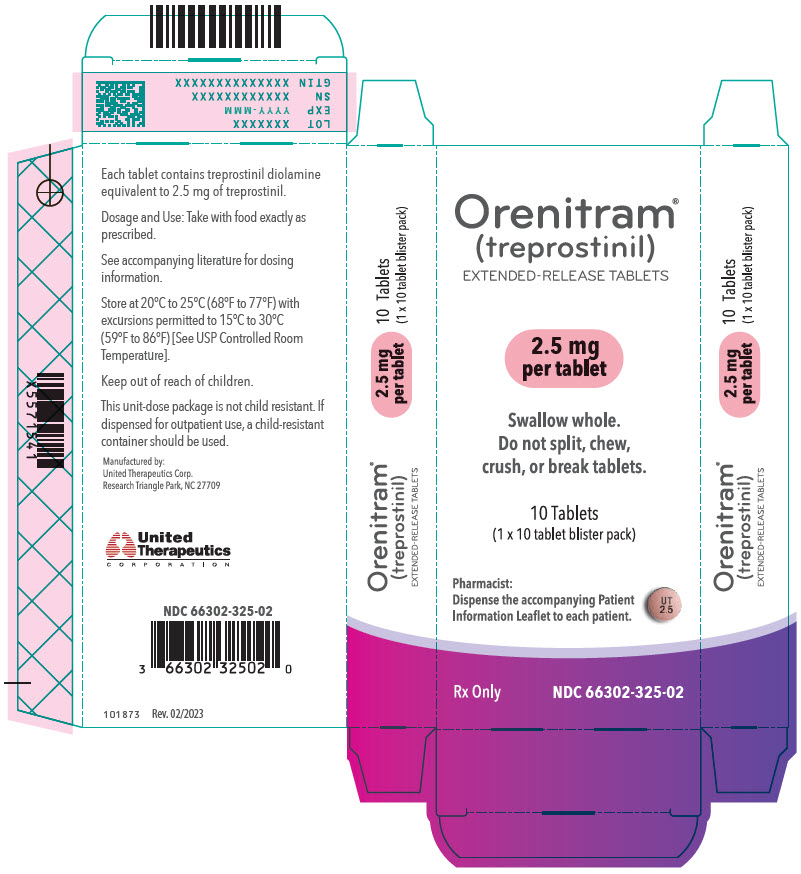
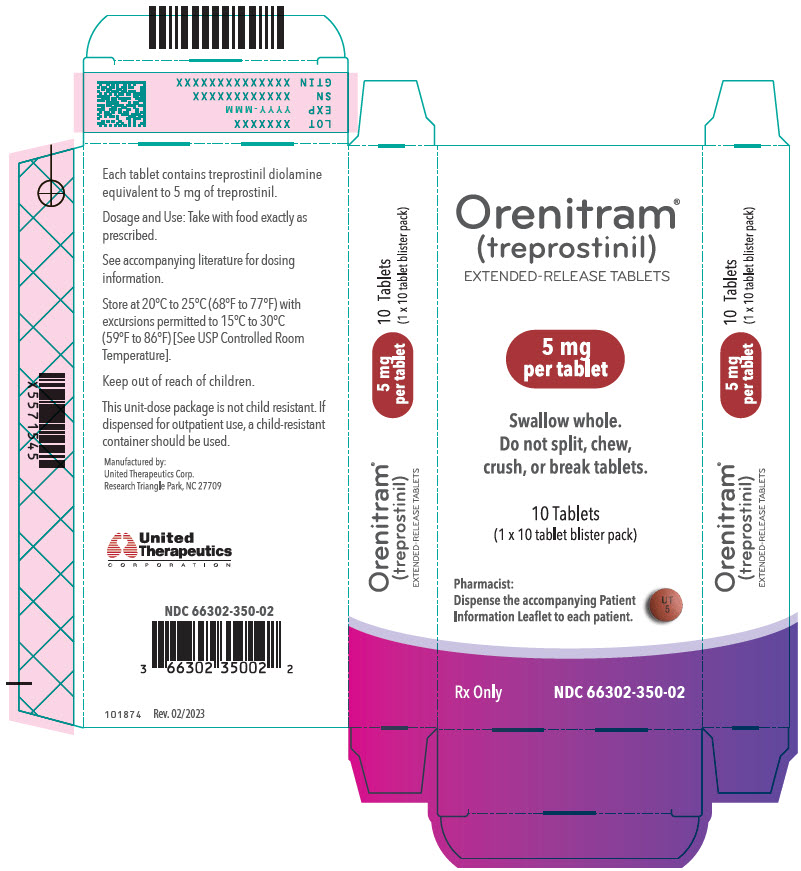
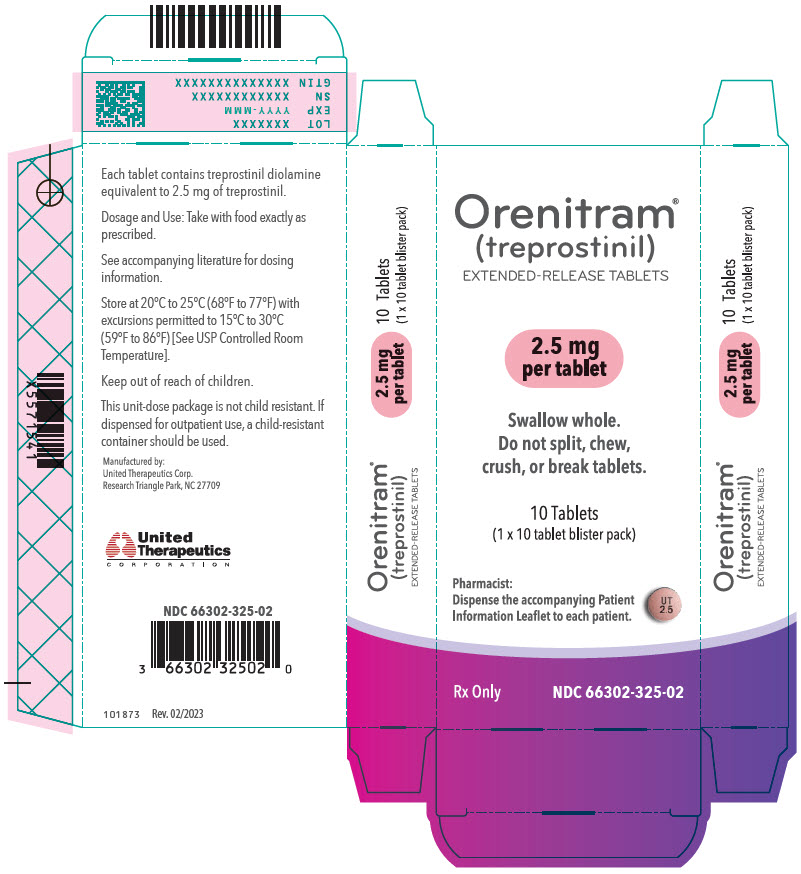
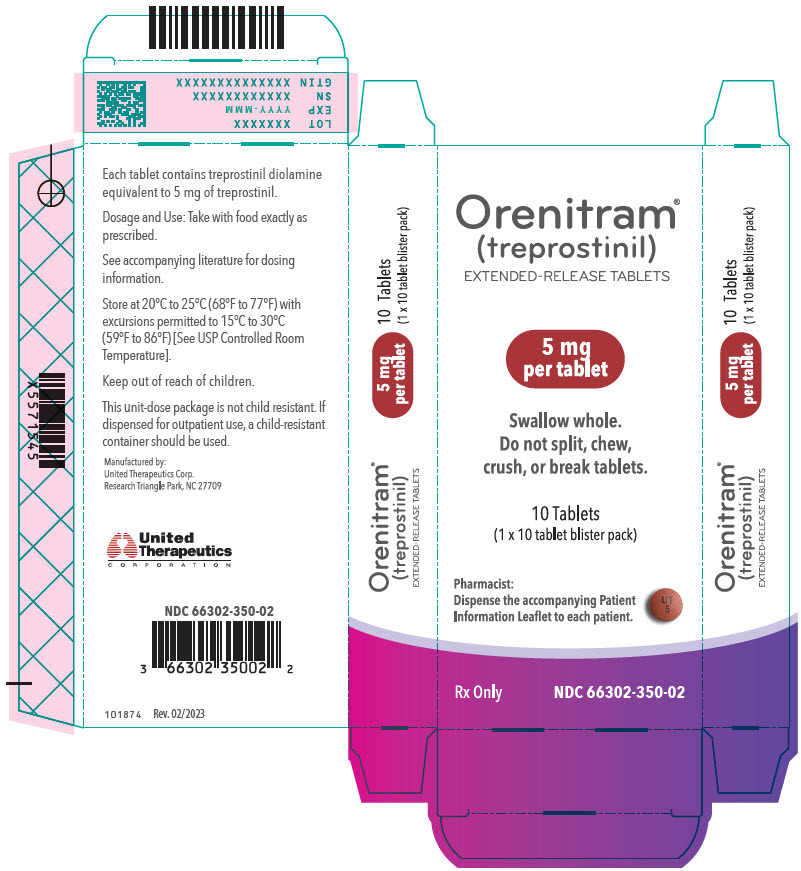
Orenitram is an 8-mm round biconvex tablet with strength identified by color and print and supplied as follows:
Strength Color Printing on Tablets NDC #
100-Count BottleNDC #
10-Count BottleNDC #
10-Count Carton (Unit-Dose Blister)0.125 mg White UT 0.125 66302-300-01 66302-300-10 66302-300-02 0.25 mg Green UT 0.25 66302-302-01 66302-302-10 66302-302-02 1 mg Yellow UT 1 66302-310-01 66302-310-10 66302-310-02 2.5 mg Pink UT 2.5 66302-325-01 66302-325-10 66302-325-02 5 mg Red UT 5 66302-350-01 66302-350-10 66302-350-02 Titration Kits for Orenitram are supplied in the following configurations:
Titration Kit Blister Configurations NDC # Month 1 containing four weekly cartons Seven daily wallet blister packs in each weekly carton containing the following total number of tablets: - Week 1 (twenty-one × 0.125 mg tablets)
- Week 2 (forty-two × 0.125 mg tablets)
- Week 3 (twenty-one × 0.125 mg tablets and twenty-one × 0.25 mg tablets)
- Week 4 (forty-two × 0.125 mg tablets and twenty-one × 0.25 mg tablets)
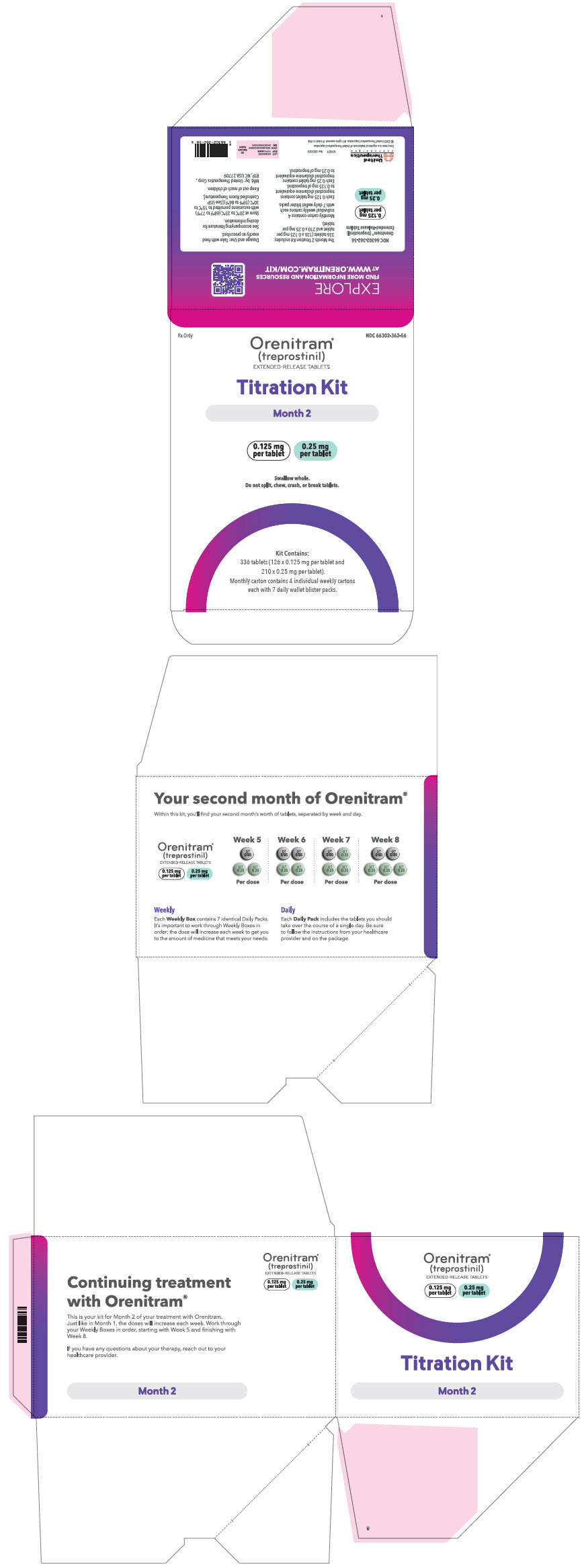
66302-361-28 Month 2 containing four weekly cartons Seven daily wallet blister packs in each weekly carton containing the following total number of tablets: - Week 5 (twenty-one × 0.125 mg tablets and forty-two × 0.25 mg tablets)
- Week 6 (forty-two × 0.125 mg tablets and forty-two × 0.25 mg tablets)
- Week 7 (twenty-one × 0.125 mg tablets and sixty-three × 0.25 mg tablets)
- Week 8 (forty-two × 0.125 mg tablets and sixty-three × 0.25 mg tablets)
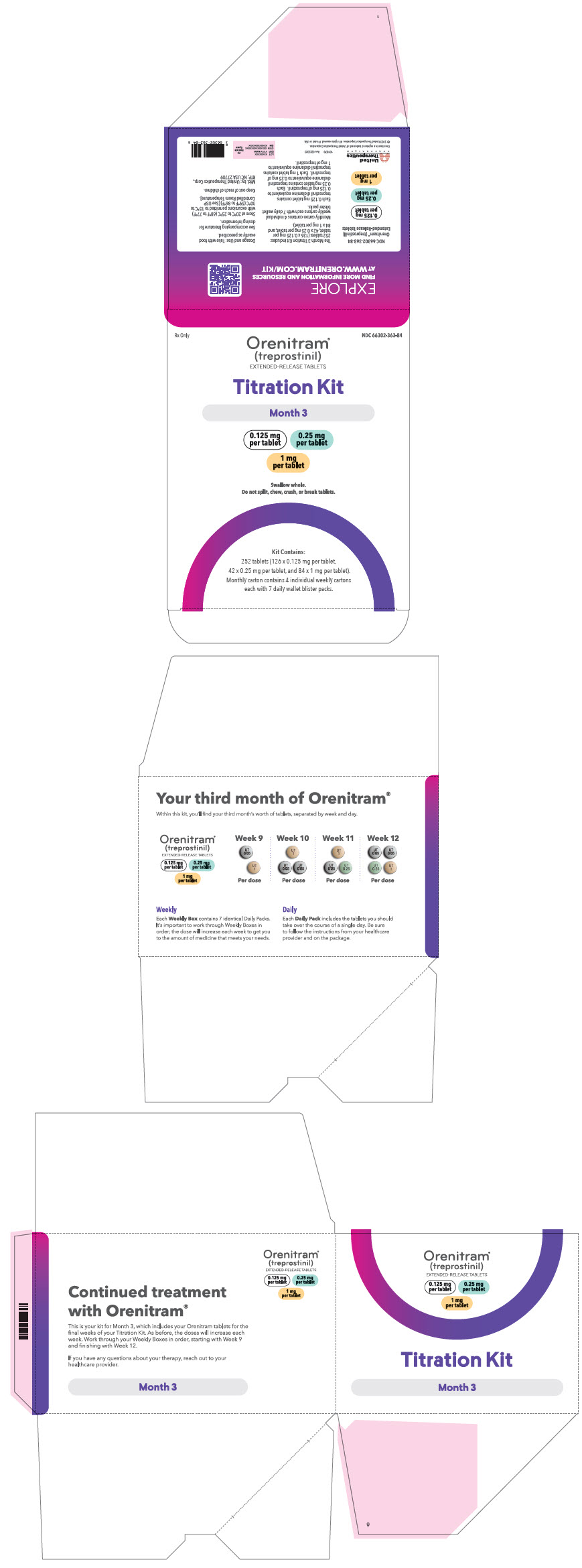
66302-362-56 Month 3 containing four weekly cartons Seven daily wallet blister packs in each weekly carton containing the following total number of tablets: - Week 9 (twenty-one × 0.125 mg tablets and twenty-one × 1 mg tablets)
- Week 10 (forty-two × 0.125 mg tablets and twenty-one × 1 mg tablets)
- Week 11 (twenty-one × 0.125 mg tablets, twenty-one × 0.25 mg tablets, and twenty-one × 1 mg tablets)
- Week 12 (forty-two × 0.125 mg tablets, twenty-one × 0.25 mg tablets, and twenty-one × 1 mg tablets)
66302-363-84 -
17 PATIENT COUNSELING INFORMATION
See FDA-approved patient labeling (Patient Information).
Tell patients:
- Abrupt discontinuation of therapy could result in worsening of PAH symptoms.
- Take Orenitram with food.
- Swallow Orenitram tablets whole. Do not split, chew, crush, or break. Do not take a tablet that is damaged or broken.
- The biologically inert components of the tablet remain intact during gastrointestinal transit and are eliminated in the feces as an insoluble shell.
- SPL UNCLASSIFIED SECTION
-
PATIENT PACKAGE INSERT
This Patient Information has been approved by the U.S. Food and Drug Administration. Revised: 02/2023 Patient Information
Orenitram(®) (oh-REN-i-tram)
(treprostinil) extended-release tabletsWhat is Orenitram? - Orenitram is a prescription medicine used to treat pulmonary arterial hypertension (PAH) which is high blood pressure in the arteries of your lungs.
- Orenitram can help slow down the progression of your disease and improve your ability to exercise.
Do not take Orenitram if you have severe liver problems. Before taking Orenitram, tell your healthcare provider about all of your medical conditions, including if you: - have liver problems
- have diverticulosis
- are pregnant or plan to become pregnant. It is not known if Orenitram will harm your unborn baby.
- are breastfeeding or plan to breastfeed. It is not known if Orenitram passes into your breast milk. Talk to your healthcare provider about the best way to feed your baby during treatment with Orenitram.
Especially tell your healthcare provider if you take another medicine that contains treprostinil, such as Remodulin®, Tyvaso®, or Tyvaso DPI®.How should I take Orenitram? - Take Orenitram exactly as your healthcare provider tells you to take it.
- Your healthcare provider will slowly increase your dose to find the dose of Orenitram that is right for you.
- If you take the medicine Remodulin and your healthcare provider is switching you to Orenitram, your healthcare provider will decrease your dose of Remodulin over a period of time when you start taking Orenitram.
- Do not change your dose or suddenly stop taking Orenitram without first talking to your healthcare provider.
- Orenitram is usually taken 3 times a day (about every 8 hours) or 2 times a day (about every 12 hours). Your healthcare provider will tell you how often you should take Orenitram. If you have side effects, your healthcare provider may tell you to change your dose or when you take Orenitram.
- Take Orenitram with food.
- Swallow Orenitram tablets whole. Do not split, chew, crush, or break your Orenitram tablets. Do not take Orenitram tablets that are damaged or broken. If Orenitram tablets are not taken whole, they may release too much medicine at one time. This can lead to side effects.
- You may see the tablet shell in your stools (bowel movements). This is usually normal. The tablet shell is not digested. If you have diverticulosis, the tablet shell may get stuck in a blind pouch or diverticulum in your intestine.
- If you miss your dose of Orenitram, take the missed dose as soon as possible with food.
- If you miss 2 or more doses of Orenitram, call your healthcare provider to see if you need to change your dose.
- If you take too much Orenitram, call your healthcare provider or go to the nearest hospital emergency room right away.
What are the possible side effects of Orenitram?
Orenitram can cause serious side effects, including worsening of PAH symptoms.- Stopping Orenitram suddenly may cause worsening of your PAH symptoms. Do not change your dose or suddenly stop taking Orenitram without first talking to your healthcare provider.
- headache
- diarrhea
- nausea
- vomiting
- flushing
- pain in arms and legs
- jaw pain
Tell your healthcare provider if you have any side effect that bothers you or that does not go away.
These are not all of the possible side effects of Orenitram. For more information, ask your healthcare provider or pharmacist.
Call your healthcare provider for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088.How should I store Orenitram? - Store at 68°F to 77°F (20°C to 25°C).
General information about the safe and effective use of Orenitram.
Medicines are sometimes prescribed for purposes other than those listed in a Patient Information Leaflet.
Do not use Orenitram for a condition for which it was not prescribed. Do not give Orenitram to other people, even if they have the same symptoms that you have. It may harm them. You can ask your healthcare provider or pharmacist for information about Orenitram that is written for health professionals.What are the ingredients in Orenitram?
Active ingredient: treprostinil diolamine
Inactive ingredients: xylitol, maltodextrin, sodium lauryl sulfate, magnesium stearate, cellulose acetate, triethyl citrate, polyvinyl alcohol, titanium dioxide, polyethylene glycol, and talc. In addition, tablets may contain colorants FD&C Blue #2, iron oxide yellow, and iron oxide red. The imprint ink contains shellac glaze, ethanol, isopropyl alcohol, iron oxide black, n-butyl alcohol, and propylene glycol.
United Therapeutics Corp., Research Triangle Park, NC 27709 USA
Copyright 2023, United Therapeutics Corp. All rights reserved.
ORENITRAM is a registered trademark of United Therapeutics Corp.
For more information, go to www.orenitram.com or call 1-877-864-8437. - PRINCIPAL DISPLAY PANEL - 0.125 mg Tablet Bottle Label
- PRINCIPAL DISPLAY PANEL - 0.125 mg Tablet Blister Pack Carton
- PRINCIPAL DISPLAY PANEL - 0.25 mg Tablet Bottle Label
- PRINCIPAL DISPLAY PANEL - 0.25 mg Tablet Blister Pack Carton
- PRINCIPAL DISPLAY PANEL - 1 mg Tablet Bottle Label
- PRINCIPAL DISPLAY PANEL - 1 mg Tablet Blister Pack Carton
- PRINCIPAL DISPLAY PANEL - 2.5 mg Tablet Bottle Label
- PRINCIPAL DISPLAY PANEL - 2.5 mg Tablet Blister Pack Carton
- PRINCIPAL DISPLAY PANEL - 5 mg Tablet Bottle Label
- PRINCIPAL DISPLAY PANEL - 5 mg Tablet Blister Pack Carton
-
PRINCIPAL DISPLAY PANEL - 0.125 mg 0.25 mg Titration Kit Carton - Month 1
Rx Only
NDC 66302-361-28
Orenitram®
(treprostinil)
EXTENDED-RELEASE TABLETSTitration Kit
Month 1
0.125 mg
per tablet0.25 mg
per tabletSwallow whole.
Do not split, chew, crush, or break tablets.Kit Contains:
168 tablets (126 x 0.125 mg per tablet and
42 x 0.25 mg per tablet).
Monthly carton contains 4 individual weekly cartons
each with 7 daily wallet blister packs.
-
PRINCIPAL DISPLAY PANEL - 0.125 mg 0.25 mg Titration Kit Carton - Month 2
Rx Only
NDC 66302-362-56
Orenitram®
(treprostinil)
EXTENDED-RELEASE TABLETSTitration Kit
Month 2
0.125 mg
per tablet0.25 mg
per tabletSwallow whole.
Do not split, chew, crush, or break tablets.Kit Contains:
336 tablets (126 x 0.125 mg per tablet and
210 x 0.25 mg per tablet).
Monthly carton contains 4 individual weekly cartons
each with 7 daily wallet blister packs.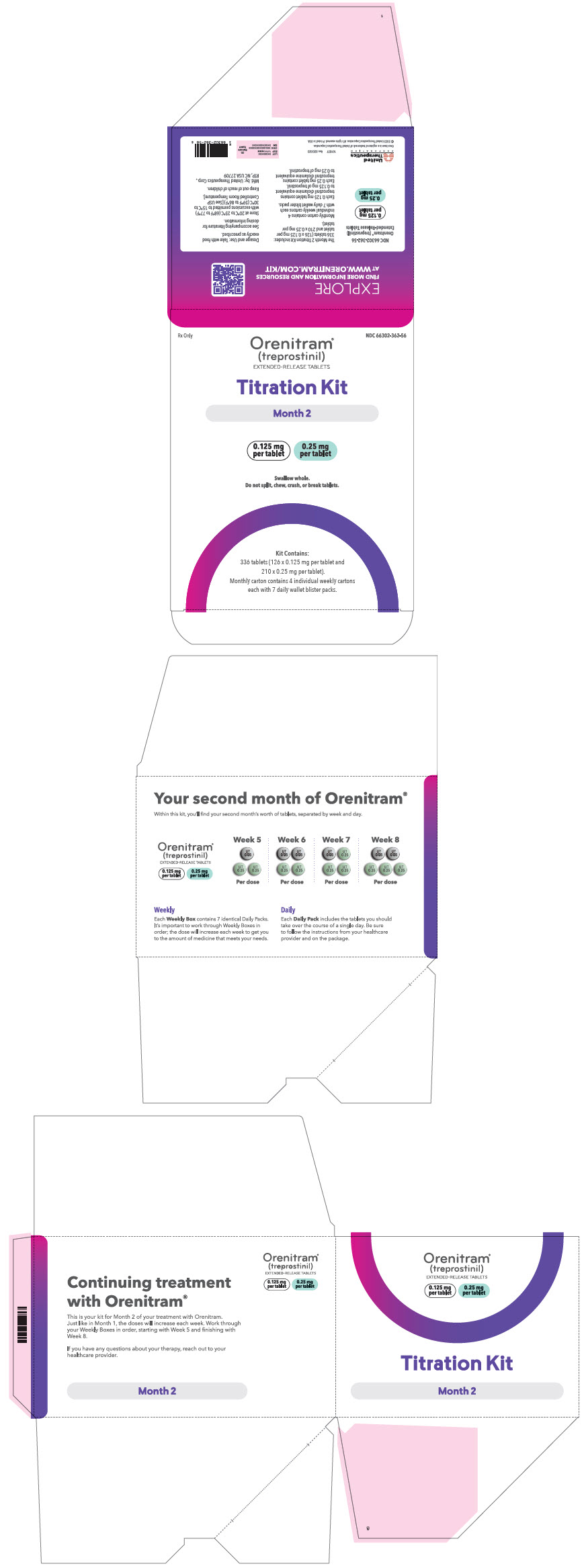
-
PRINCIPAL DISPLAY PANEL - 0.125 mg 0.25 mg 1 mg Titration Kit Carton - Month 3
Rx Only
NDC 66302-363-84
Orenitram®
(treprostinil)
EXTENDED-RELEASE TABLETSTitration Kit
Month 3
0.125 mg
per tablet0.25 mg
per tablet1 mg
per tabletSwallow whole.
Do not split, chew, crush, or break tablets.Kit Contains:
252 tablets (126 x 0.125 mg per tablet,
42 x 0.25 mg per tablet, and 84 × 1 mg per tablet).
Monthly carton contains 4 individual weekly cartons
each with 7 daily wallet blister packs.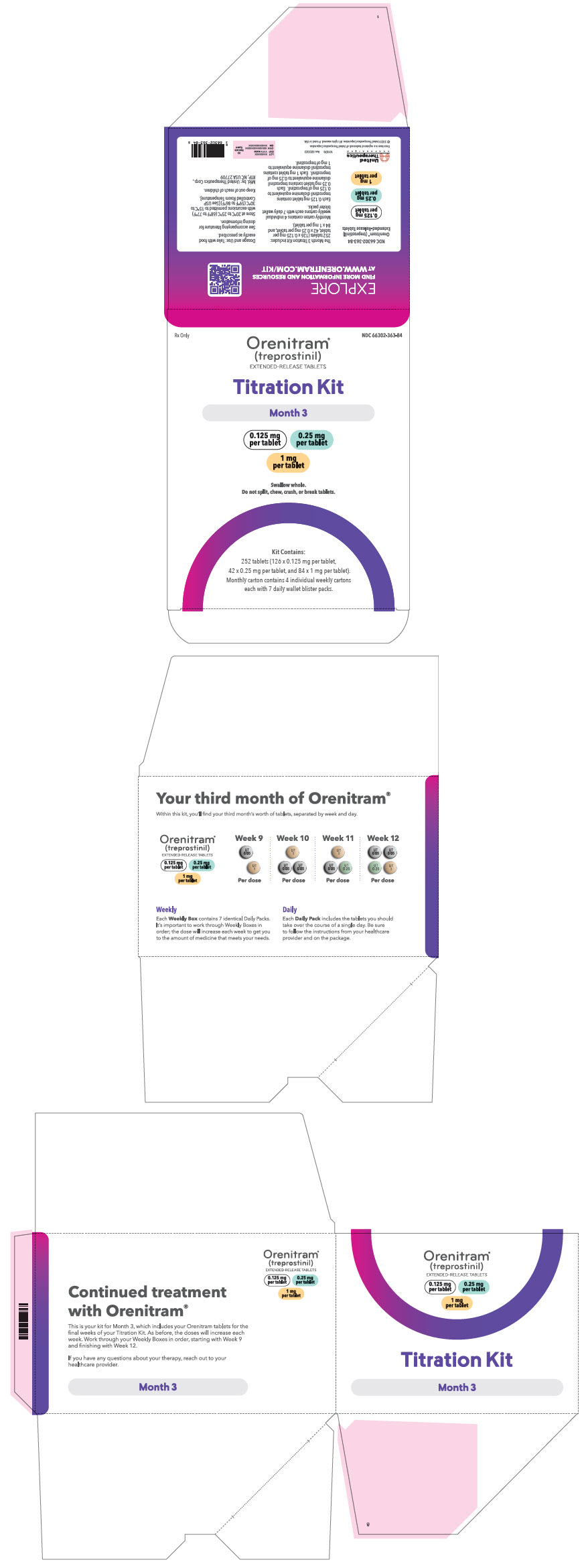
-
INGREDIENTS AND APPEARANCE
ORENITRAM
treprostinil tablet, extended releaseProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:66302-300 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength treprostinil (UNII: RUM6K67ESG) (treprostinil - UNII:RUM6K67ESG) treprostinil 0.125 mg Inactive Ingredients Ingredient Name Strength Xylitol (UNII: VCQ006KQ1E) Maltodextrin (UNII: 7CVR7L4A2D) Sodium Lauryl Sulfate (UNII: 368GB5141J) Magnesium Stearate (UNII: 70097M6I30) Cellulose Acetate (UNII: 3J2P07GVB6) Triethyl Citrate (UNII: 8Z96QXD6UM) Polyvinyl alcohol, unspecified (UNII: 532B59J990) Titanium Dioxide (UNII: 15FIX9V2JP) Polyethylene Glycol 3350 (UNII: G2M7P15E5P) Talc (UNII: 7SEV7J4R1U) Shellac (UNII: 46N107B71O) Ferrosoferric Oxide (UNII: XM0M87F357) BUTYL ALCOHOL (UNII: 8PJ61P6TS3) Alcohol (UNII: 3K9958V90M) Isopropyl Alcohol (UNII: ND2M416302) Propylene Glycol (UNII: 6DC9Q167V3) Product Characteristics Color WHITE Score no score Shape ROUND (Biconvex) Size 8mm Flavor Imprint Code UT;0;125 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:66302-300-01 100 in 1 BOTTLE; Type 0: Not a Combination Product 12/20/2013 2 NDC:66302-300-10 10 in 1 BOTTLE; Type 0: Not a Combination Product 12/20/2013 01/31/2024 3 NDC:66302-300-02 1 in 1 CARTON 06/30/2021 3 10 in 1 BLISTER PACK; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA203496 12/20/2013 ORENITRAM
treprostinil tablet, extended releaseProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:66302-302 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength treprostinil (UNII: RUM6K67ESG) (treprostinil - UNII:RUM6K67ESG) treprostinil 0.25 mg Inactive Ingredients Ingredient Name Strength Xylitol (UNII: VCQ006KQ1E) Maltodextrin (UNII: 7CVR7L4A2D) Sodium Lauryl Sulfate (UNII: 368GB5141J) Magnesium Stearate (UNII: 70097M6I30) Cellulose Acetate (UNII: 3J2P07GVB6) Triethyl Citrate (UNII: 8Z96QXD6UM) Polyvinyl alcohol, unspecified (UNII: 532B59J990) Titanium Dioxide (UNII: 15FIX9V2JP) Polyethylene Glycol 3350 (UNII: G2M7P15E5P) Talc (UNII: 7SEV7J4R1U) FD&C Blue No. 2 (UNII: L06K8R7DQK) Ferric Oxide Yellow (UNII: EX438O2MRT) Shellac (UNII: 46N107B71O) Ferrosoferric Oxide (UNII: XM0M87F357) BUTYL ALCOHOL (UNII: 8PJ61P6TS3) Alcohol (UNII: 3K9958V90M) Isopropyl Alcohol (UNII: ND2M416302) Propylene Glycol (UNII: 6DC9Q167V3) Product Characteristics Color GREEN Score no score Shape ROUND (Biconvex) Size 8mm Flavor Imprint Code UT;0;25 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:66302-302-01 100 in 1 BOTTLE; Type 0: Not a Combination Product 12/20/2013 2 NDC:66302-302-10 10 in 1 BOTTLE; Type 0: Not a Combination Product 12/20/2013 02/29/2024 3 NDC:66302-302-02 1 in 1 CARTON 06/30/2021 3 10 in 1 BLISTER PACK; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA203496 12/20/2013 ORENITRAM
treprostinil tablet, extended releaseProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:66302-310 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength treprostinil (UNII: RUM6K67ESG) (treprostinil - UNII:RUM6K67ESG) treprostinil 1 mg Inactive Ingredients Ingredient Name Strength Xylitol (UNII: VCQ006KQ1E) Maltodextrin (UNII: 7CVR7L4A2D) Sodium Lauryl Sulfate (UNII: 368GB5141J) Magnesium Stearate (UNII: 70097M6I30) Cellulose Acetate (UNII: 3J2P07GVB6) Triethyl Citrate (UNII: 8Z96QXD6UM) Polyvinyl alcohol, unspecified (UNII: 532B59J990) Titanium Dioxide (UNII: 15FIX9V2JP) Polyethylene Glycol 3350 (UNII: G2M7P15E5P) Talc (UNII: 7SEV7J4R1U) Ferric Oxide Yellow (UNII: EX438O2MRT) Ferric Oxide Red (UNII: 1K09F3G675) Shellac (UNII: 46N107B71O) Ferrosoferric Oxide (UNII: XM0M87F357) BUTYL ALCOHOL (UNII: 8PJ61P6TS3) Alcohol (UNII: 3K9958V90M) Isopropyl Alcohol (UNII: ND2M416302) Propylene Glycol (UNII: 6DC9Q167V3) Product Characteristics Color YELLOW Score no score Shape ROUND (Biconvex) Size 8mm Flavor Imprint Code UT;1 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:66302-310-01 100 in 1 BOTTLE; Type 0: Not a Combination Product 12/20/2013 2 NDC:66302-310-10 10 in 1 BOTTLE; Type 0: Not a Combination Product 12/20/2013 02/29/2024 3 NDC:66302-310-02 1 in 1 CARTON 06/30/2021 3 10 in 1 BLISTER PACK; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA203496 12/20/2013 ORENITRAM
treprostinil tablet, extended releaseProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:66302-325 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength treprostinil (UNII: RUM6K67ESG) (treprostinil - UNII:RUM6K67ESG) treprostinil 2.5 mg Inactive Ingredients Ingredient Name Strength Xylitol (UNII: VCQ006KQ1E) Maltodextrin (UNII: 7CVR7L4A2D) Sodium Lauryl Sulfate (UNII: 368GB5141J) Magnesium Stearate (UNII: 70097M6I30) Cellulose Acetate (UNII: 3J2P07GVB6) Triethyl Citrate (UNII: 8Z96QXD6UM) Polyvinyl alcohol, unspecified (UNII: 532B59J990) Titanium Dioxide (UNII: 15FIX9V2JP) Polyethylene Glycol 3350 (UNII: G2M7P15E5P) Talc (UNII: 7SEV7J4R1U) Ferric Oxide Red (UNII: 1K09F3G675) Shellac (UNII: 46N107B71O) Ferrosoferric Oxide (UNII: XM0M87F357) BUTYL ALCOHOL (UNII: 8PJ61P6TS3) Alcohol (UNII: 3K9958V90M) Isopropyl Alcohol (UNII: ND2M416302) Propylene Glycol (UNII: 6DC9Q167V3) Product Characteristics Color PINK Score no score Shape ROUND (Biconvex) Size 8mm Flavor Imprint Code UT;2;5 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:66302-325-01 100 in 1 BOTTLE; Type 0: Not a Combination Product 12/20/2013 2 NDC:66302-325-10 10 in 1 BOTTLE; Type 0: Not a Combination Product 12/20/2013 02/29/2024 3 NDC:66302-325-02 1 in 1 CARTON 06/30/2021 3 10 in 1 BLISTER PACK; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA203496 12/20/2013 ORENITRAM
treprostinil tablet, extended releaseProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:66302-350 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength treprostinil (UNII: RUM6K67ESG) (treprostinil - UNII:RUM6K67ESG) treprostinil 5 mg Inactive Ingredients Ingredient Name Strength Xylitol (UNII: VCQ006KQ1E) Maltodextrin (UNII: 7CVR7L4A2D) Sodium Lauryl Sulfate (UNII: 368GB5141J) Magnesium Stearate (UNII: 70097M6I30) Cellulose Acetate (UNII: 3J2P07GVB6) Triethyl Citrate (UNII: 8Z96QXD6UM) Polyvinyl alcohol, unspecified (UNII: 532B59J990) Titanium Dioxide (UNII: 15FIX9V2JP) Polyethylene Glycol 3350 (UNII: G2M7P15E5P) Talc (UNII: 7SEV7J4R1U) Ferric Oxide Red (UNII: 1K09F3G675) Shellac (UNII: 46N107B71O) Ferrosoferric Oxide (UNII: XM0M87F357) BUTYL ALCOHOL (UNII: 8PJ61P6TS3) Alcohol (UNII: 3K9958V90M) Isopropyl Alcohol (UNII: ND2M416302) Propylene Glycol (UNII: 6DC9Q167V3) Product Characteristics Color RED Score no score Shape ROUND (Biconvex) Size 8mm Flavor Imprint Code UT;5 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:66302-350-01 100 in 1 BOTTLE; Type 0: Not a Combination Product 12/20/2013 2 NDC:66302-350-10 10 in 1 BOTTLE; Type 0: Not a Combination Product 12/20/2013 02/29/2024 3 NDC:66302-350-02 1 in 1 CARTON 06/30/2021 3 10 in 1 BLISTER PACK; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA203496 12/20/2013 ORENITRAM
treprostinil kitProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:66302-361 Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:66302-361-28 1 in 1 PACKAGE 02/14/2023 Quantity of Parts Part # Package Quantity Total Product Quantity Part 1 1 BLISTER PACK 126 Part 2 1 BLISTER PACK 42 Part 1 of 2 ORENITRAM
treprostinil tablet, extended releaseProduct Information Item Code (Source) NDC:66302-300 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength treprostinil (UNII: RUM6K67ESG) (treprostinil - UNII:RUM6K67ESG) treprostinil 0.125 mg Inactive Ingredients Ingredient Name Strength Xylitol (UNII: VCQ006KQ1E) Maltodextrin (UNII: 7CVR7L4A2D) Sodium Lauryl Sulfate (UNII: 368GB5141J) Magnesium Stearate (UNII: 70097M6I30) Cellulose Acetate (UNII: 3J2P07GVB6) Triethyl Citrate (UNII: 8Z96QXD6UM) Polyvinyl alcohol, unspecified (UNII: 532B59J990) Titanium Dioxide (UNII: 15FIX9V2JP) Polyethylene Glycol 3350 (UNII: G2M7P15E5P) Talc (UNII: 7SEV7J4R1U) Shellac (UNII: 46N107B71O) Ferrosoferric Oxide (UNII: XM0M87F357) Butyl Alcohol (UNII: 8PJ61P6TS3) Alcohol (UNII: 3K9958V90M) Isopropyl Alcohol (UNII: ND2M416302) Propylene Glycol (UNII: 6DC9Q167V3) Product Characteristics Color WHITE Score no score Shape ROUND (Biconvex) Size 8mm Flavor Imprint Code UT;0;125 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 126 in 1 BLISTER PACK; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA203496 02/14/2023 Part 2 of 2 ORENITRAM
treprostinil tablet, extended releaseProduct Information Item Code (Source) NDC:66302-302 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength treprostinil (UNII: RUM6K67ESG) (treprostinil - UNII:RUM6K67ESG) treprostinil 0.25 mg Inactive Ingredients Ingredient Name Strength Xylitol (UNII: VCQ006KQ1E) Maltodextrin (UNII: 7CVR7L4A2D) Sodium Lauryl Sulfate (UNII: 368GB5141J) Magnesium Stearate (UNII: 70097M6I30) Cellulose Acetate (UNII: 3J2P07GVB6) Triethyl Citrate (UNII: 8Z96QXD6UM) Polyvinyl alcohol, unspecified (UNII: 532B59J990) Titanium Dioxide (UNII: 15FIX9V2JP) Polyethylene Glycol 3350 (UNII: G2M7P15E5P) Talc (UNII: 7SEV7J4R1U) Shellac (UNII: 46N107B71O) Ferrosoferric Oxide (UNII: XM0M87F357) Butyl Alcohol (UNII: 8PJ61P6TS3) Alcohol (UNII: 3K9958V90M) Isopropyl Alcohol (UNII: ND2M416302) Propylene Glycol (UNII: 6DC9Q167V3) Ferric Oxide Yellow (UNII: EX438O2MRT) FD&C BLUE NO. 2 (UNII: L06K8R7DQK) Product Characteristics Color GREEN Score no score Shape ROUND (Biconvex) Size 8mm Flavor Imprint Code UT;0;25 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 42 in 1 BLISTER PACK; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA203496 02/14/2023 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA203496 02/14/2023 ORENITRAM
treprostinil kitProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:66302-362 Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:66302-362-56 1 in 1 PACKAGE 02/14/2023 Quantity of Parts Part # Package Quantity Total Product Quantity Part 1 1 BLISTER PACK 126 Part 2 1 BLISTER PACK 210 Part 1 of 2 ORENITRAM
treprostinil tablet, extended releaseProduct Information Item Code (Source) NDC:66302-300 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength treprostinil (UNII: RUM6K67ESG) (treprostinil - UNII:RUM6K67ESG) treprostinil 0.125 mg Inactive Ingredients Ingredient Name Strength Xylitol (UNII: VCQ006KQ1E) Maltodextrin (UNII: 7CVR7L4A2D) Sodium Lauryl Sulfate (UNII: 368GB5141J) Magnesium Stearate (UNII: 70097M6I30) Cellulose Acetate (UNII: 3J2P07GVB6) Triethyl Citrate (UNII: 8Z96QXD6UM) Polyvinyl alcohol, unspecified (UNII: 532B59J990) Titanium Dioxide (UNII: 15FIX9V2JP) Polyethylene Glycol 3350 (UNII: G2M7P15E5P) Talc (UNII: 7SEV7J4R1U) Shellac (UNII: 46N107B71O) Ferrosoferric Oxide (UNII: XM0M87F357) Butyl Alcohol (UNII: 8PJ61P6TS3) Alcohol (UNII: 3K9958V90M) Isopropyl Alcohol (UNII: ND2M416302) Propylene Glycol (UNII: 6DC9Q167V3) Product Characteristics Color WHITE Score no score Shape ROUND (Biconvex) Size 8mm Flavor Imprint Code UT;0;125 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 126 in 1 BLISTER PACK; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA203496 02/14/2023 Part 2 of 2 ORENITRAM
treprostinil tablet, extended releaseProduct Information Item Code (Source) NDC:66302-302 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength treprostinil (UNII: RUM6K67ESG) (treprostinil - UNII:RUM6K67ESG) treprostinil 0.25 mg Inactive Ingredients Ingredient Name Strength Xylitol (UNII: VCQ006KQ1E) Maltodextrin (UNII: 7CVR7L4A2D) Sodium Lauryl Sulfate (UNII: 368GB5141J) Magnesium Stearate (UNII: 70097M6I30) Cellulose Acetate (UNII: 3J2P07GVB6) Triethyl Citrate (UNII: 8Z96QXD6UM) Polyvinyl alcohol, unspecified (UNII: 532B59J990) Titanium Dioxide (UNII: 15FIX9V2JP) Polyethylene Glycol 3350 (UNII: G2M7P15E5P) Talc (UNII: 7SEV7J4R1U) Shellac (UNII: 46N107B71O) Ferrosoferric Oxide (UNII: XM0M87F357) Butyl Alcohol (UNII: 8PJ61P6TS3) Alcohol (UNII: 3K9958V90M) Isopropyl Alcohol (UNII: ND2M416302) Propylene Glycol (UNII: 6DC9Q167V3) Ferric Oxide Yellow (UNII: EX438O2MRT) FD&C BLUE NO. 2 (UNII: L06K8R7DQK) Product Characteristics Color GREEN Score no score Shape ROUND (Biconvex) Size 8mm Flavor Imprint Code UT;0;25 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 210 in 1 BLISTER PACK; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA203496 02/14/2023 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA203496 02/14/2023 ORENITRAM
treprostinil kitProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:66302-363 Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:66302-363-84 1 in 1 PACKAGE 02/14/2023 Quantity of Parts Part # Package Quantity Total Product Quantity Part 1 1 BLISTER PACK 126 Part 2 1 BLISTER PACK 42 Part 3 1 BLISTER PACK 84 Part 1 of 3 ORENITRAM
treprostinil tablet, extended releaseProduct Information Item Code (Source) NDC:66302-300 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength treprostinil (UNII: RUM6K67ESG) (treprostinil - UNII:RUM6K67ESG) treprostinil 0.125 mg Inactive Ingredients Ingredient Name Strength Xylitol (UNII: VCQ006KQ1E) Maltodextrin (UNII: 7CVR7L4A2D) Sodium Lauryl Sulfate (UNII: 368GB5141J) Magnesium Stearate (UNII: 70097M6I30) Cellulose Acetate (UNII: 3J2P07GVB6) Triethyl Citrate (UNII: 8Z96QXD6UM) Polyvinyl alcohol, unspecified (UNII: 532B59J990) Titanium Dioxide (UNII: 15FIX9V2JP) Polyethylene Glycol 3350 (UNII: G2M7P15E5P) Talc (UNII: 7SEV7J4R1U) Shellac (UNII: 46N107B71O) Ferrosoferric Oxide (UNII: XM0M87F357) Butyl Alcohol (UNII: 8PJ61P6TS3) Alcohol (UNII: 3K9958V90M) Isopropyl Alcohol (UNII: ND2M416302) Propylene Glycol (UNII: 6DC9Q167V3) Product Characteristics Color WHITE Score no score Shape ROUND (Biconvex) Size 8mm Flavor Imprint Code UT;0;125 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 126 in 1 BLISTER PACK; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA203496 02/14/2023 Part 2 of 3 ORENITRAM
treprostinil tablet, extended releaseProduct Information Item Code (Source) NDC:66302-302 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength treprostinil (UNII: RUM6K67ESG) (treprostinil - UNII:RUM6K67ESG) treprostinil 0.25 mg Inactive Ingredients Ingredient Name Strength Xylitol (UNII: VCQ006KQ1E) Maltodextrin (UNII: 7CVR7L4A2D) Sodium Lauryl Sulfate (UNII: 368GB5141J) Magnesium Stearate (UNII: 70097M6I30) Cellulose Acetate (UNII: 3J2P07GVB6) Triethyl Citrate (UNII: 8Z96QXD6UM) Polyvinyl alcohol, unspecified (UNII: 532B59J990) Titanium Dioxide (UNII: 15FIX9V2JP) Polyethylene Glycol 3350 (UNII: G2M7P15E5P) Talc (UNII: 7SEV7J4R1U) Shellac (UNII: 46N107B71O) Ferrosoferric Oxide (UNII: XM0M87F357) Butyl Alcohol (UNII: 8PJ61P6TS3) Alcohol (UNII: 3K9958V90M) Isopropyl Alcohol (UNII: ND2M416302) Propylene Glycol (UNII: 6DC9Q167V3) Ferric Oxide Yellow (UNII: EX438O2MRT) FD&C BLUE NO. 2 (UNII: L06K8R7DQK) Product Characteristics Color GREEN Score no score Shape ROUND (Biconvex) Size 8mm Flavor Imprint Code UT;0;25 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 42 in 1 BLISTER PACK; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA203496 02/14/2023 Part 3 of 3 ORENITRAM
treprostinil tablet, extended releaseProduct Information Item Code (Source) NDC:66302-310 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength treprostinil (UNII: RUM6K67ESG) (treprostinil - UNII:RUM6K67ESG) treprostinil 1 mg Inactive Ingredients Ingredient Name Strength Xylitol (UNII: VCQ006KQ1E) Maltodextrin (UNII: 7CVR7L4A2D) Sodium Lauryl Sulfate (UNII: 368GB5141J) Magnesium Stearate (UNII: 70097M6I30) Cellulose Acetate (UNII: 3J2P07GVB6) Triethyl Citrate (UNII: 8Z96QXD6UM) Polyvinyl alcohol, unspecified (UNII: 532B59J990) Titanium Dioxide (UNII: 15FIX9V2JP) Polyethylene Glycol 3350 (UNII: G2M7P15E5P) Talc (UNII: 7SEV7J4R1U) Shellac (UNII: 46N107B71O) Ferrosoferric Oxide (UNII: XM0M87F357) Butyl Alcohol (UNII: 8PJ61P6TS3) Alcohol (UNII: 3K9958V90M) Isopropyl Alcohol (UNII: ND2M416302) Propylene Glycol (UNII: 6DC9Q167V3) Ferric Oxide Yellow (UNII: EX438O2MRT) Ferric Oxide Red (UNII: 1K09F3G675) Product Characteristics Color YELLOW Score no score Shape ROUND (Biconvex) Size 8mm Flavor Imprint Code UT;1 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 84 in 1 BLISTER PACK; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA203496 02/14/2023 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA203496 02/14/2023 Labeler - United Therapeutics Corporation (965460025) Establishment Name Address ID/FEI Business Operations United Therapeutics Corporation 015718364 MANUFACTURE(66302-300, 66302-302, 66302-310, 66302-325, 66302-350, 66302-361, 66302-362, 66302-363) , ANALYSIS(66302-300, 66302-302, 66302-310, 66302-325, 66302-350, 66302-361, 66302-362, 66302-363) , LABEL(66302-300, 66302-302, 66302-310, 66302-325, 66302-350, 66302-361, 66302-362, 66302-363) , PACK(66302-300, 66302-302, 66302-310, 66302-325, 66302-350, 66302-361, 66302-362, 66302-363)