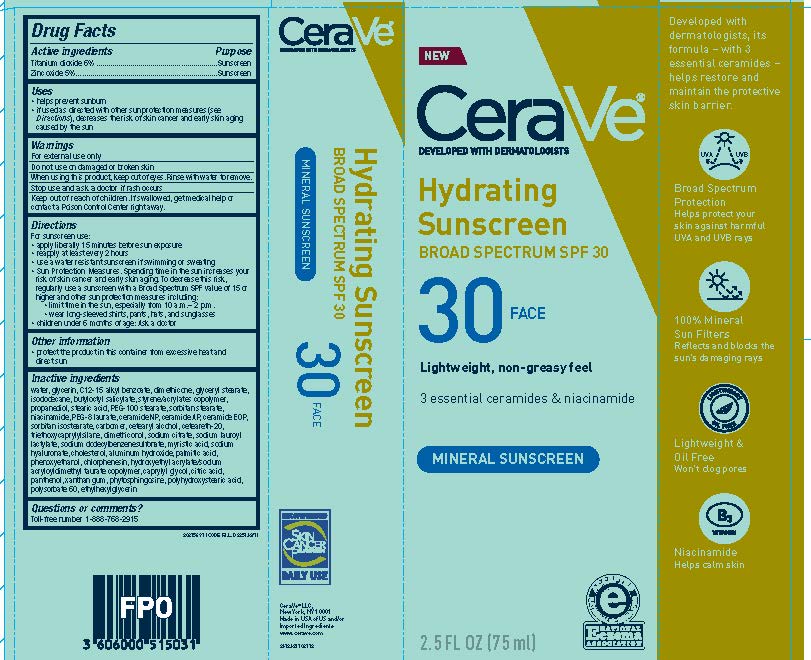
Label: CERAVE DEVELOPED WITH DERMATOLOGISTS HYDRATING SUNSCREEN SPF 30 FACE LIGHTWEIGHT, NON-GREASY FEEL 3 ESSENTIAL CERAMIDES NIACINAMIDE MINERAL SUNSCREEN- titanium dioxide and zinc oxide lotion
- NDC Code(s): 49967-150-01, 49967-150-02, 49967-150-03
- Packager: L'Oreal USA Products Inc
- Category: HUMAN OTC DRUG LABEL
Drug Label Information
Updated December 31, 2023
If you are a healthcare professional or from the pharmaceutical industry please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Active ingredients
- Purpose
- Uses
- Warnings
- Do not use
- Stop use and ask a doctor if
- Keep out of reach of children.
-
Directions
For sunscreen use:
- apply liberally 15 minutes before sun exposure
- reapply at least every 2 hours
- use a water resistant sunscreen if swimming or sweating
- Sun Protection Measures. Spending time in the sun increases your risk of skin cancer and early skin aging. To decrease this risk, regularly use a sunscreen with a Broad Spectrum SPF value of 15 or higher and other sun protection measures including:
- limit time in the sun, especially from 10 a.m.-2p.m.
- wear long-sleeved shirts, pants, hats and sunglasses
- children under 6 months of age: Ask a doctor
- Other information
-
Inactive ingredients
water, glycerin, C12-15 alkyl benzoate, dimethicone, glyceryl stearate, isododecane, butyloctyl salicylate, styrene/acrylates copolymer, propanediol, stearic acid, PEG-100 sterate, sorbitan stearate, niacinamide, acrylates/dimethicone copolymer, PEG-8 laurate, ceramide NP, ceramide AP, ceramide EOP, sorbitan isostearate, carbomer, cetearyl alcohol, ceteareth-20, triethoxycaprylylsilane, dimethiconol, sodium citrate, sodium lauroyl lactylate, sodium dodecylbenzenesulfonate, myristic acid, sodium hyaluronate, cholesterol, aluminum hydroxide, palmitic acid, phenoxyethanol, chlorphenesin, hydroxyethyl acrylate/sodium acryloyldimethyl taurate copolymer, caprylyl glycol, citric acid, panthenol, xanthan gum, phytosphingosine, polyhydroxystearic acid, polysorbate 60, ethylhexylglycerin
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
CERAVE DEVELOPED WITH DERMATOLOGISTS HYDRATING SUNSCREEN SPF 30 FACE LIGHTWEIGHT, NON-GREASY FEEL 3 ESSENTIAL CERAMIDES NIACINAMIDE MINERAL SUNSCREEN
titanium dioxide and zinc oxide lotionProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:49967-150 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength TITANIUM DIOXIDE (UNII: 15FIX9V2JP) (TITANIUM DIOXIDE - UNII:15FIX9V2JP) TITANIUM DIOXIDE 60 mg in 1 mL ZINC OXIDE (UNII: SOI2LOH54Z) (ZINC CATION - UNII:13S1S8SF37) ZINC CATION 50 mg in 1 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) GLYCERIN (UNII: PDC6A3C0OX) ALKYL (C12-15) BENZOATE (UNII: A9EJ3J61HQ) DIMETHICONE (UNII: 92RU3N3Y1O) ISODODECANE (UNII: A8289P68Y2) GLYCERYL MONOSTEARATE (UNII: 230OU9XXE4) BUTYLOCTYL SALICYLATE (UNII: 2EH13UN8D3) PROPANEDIOL (UNII: 5965N8W85T) STEARIC ACID (UNII: 4ELV7Z65AP) PEG-100 STEARATE (UNII: YD01N1999R) SORBITAN MONOSTEARATE (UNII: NVZ4I0H58X) NIACINAMIDE (UNII: 25X51I8RD4) PEG-8 LAURATE (UNII: 762O8IWA10) CERAMIDE NP (UNII: 4370DF050B) CERAMIDE AP (UNII: F1X8L2B00J) CERAMIDE 1 (UNII: 5THT33P7X7) SORBITAN ISOSTEARATE (UNII: 01S2G2C1E4) CARBOXYPOLYMETHYLENE (UNII: 0A5MM307FC) CETOSTEARYL ALCOHOL (UNII: 2DMT128M1S) POLYOXYL 20 CETOSTEARYL ETHER (UNII: YRC528SWUY) TRIETHOXYCAPRYLYLSILANE (UNII: LDC331P08E) SODIUM CITRATE (UNII: 1Q73Q2JULR) SODIUM LAUROYL LACTYLATE (UNII: 7243K85WFO) SODIUM DODECYLBENZENESULFONATE (UNII: 554127163Y) MYRISTIC ACID (UNII: 0I3V7S25AW) HYALURONATE SODIUM (UNII: YSE9PPT4TH) CHOLESTEROL (UNII: 97C5T2UQ7J) ALUMINUM HYDROXIDE (UNII: 5QB0T2IUN0) PALMITIC ACID (UNII: 2V16EO95H1) PHENOXYETHANOL (UNII: HIE492ZZ3T) CHLORPHENESIN (UNII: I670DAL4SZ) HYDROXYETHYL ACRYLATE/SODIUM ACRYLOYLDIMETHYL TAURATE COPOLYMER (45000 MPA.S AT 1%) (UNII: 86FQE96TZ4) CAPRYLYL GLYCOL (UNII: 00YIU5438U) CITRIC ACID ACETATE (UNII: DSO12WL7AU) PANTHENOL (UNII: WV9CM0O67Z) XANTHAN GUM (UNII: TTV12P4NEE) PHYTOSPHINGOSINE (UNII: GIN46U9Q2Q) POLYHYDROXYSTEARIC ACID STEARATE (UNII: 8KQ7I65XZE) POLYSORBATE 60 (UNII: CAL22UVI4M) ETHYLHEXYLGLYCERIN (UNII: 147D247K3P) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:49967-150-01 1 in 1 CARTON 02/01/2019 1 75 mL in 1 TUBE; Type 0: Not a Combination Product 2 NDC:49967-150-02 5 mL in 1 TUBE; Type 0: Not a Combination Product 02/01/2019 3 NDC:49967-150-03 2.5 mL in 1 TUBE; Type 0: Not a Combination Product 02/01/2019 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M020 02/01/2019 Labeler - L'Oreal USA Products Inc (002136794) Establishment Name Address ID/FEI Business Operations L'OREAL USA PRODUCTS, INC. 624244349 manufacture(49967-150)