Label: STERILLIUM RUB FRAGRANCE FREE- alcohol liquid
- NDC Code(s): 65616-008-06, 65616-008-08
- Packager: BODE Chemie GmbH
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated December 11, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Active ingredient
- Purpose
- Uses
- Warnings
- DO NOT USE
- WHEN USING
- STOP USE
- KEEP OUT OF REACH OF CHILDREN
-
Directions
- apply to clean, dry hands
- for the first use of the day, use a nail pick
- dispense approx. 2 mL into hand, dip fingers of opposite hand into palm, working product under nails and into cuticles
- repeat procedure with other hand
- with hands still moist spread around the hand and lower 1/3 of the forearm
- reapply the product to the hands, paying particular attention to fingers, cuticles, and interdigital spaces
- following application, rub hands until dry
- hands should remain moist for entire application time, approx. 1.5 minutes.
- Inactive ingredients
-
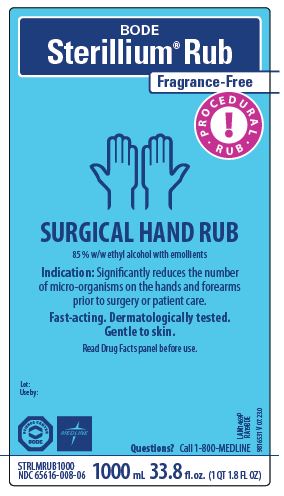
PRINCIPAL DISPLAY PANEL
BODE
Sterillium Rub
Fragrance-Free
Procedural Rub
SURGICAL HAND RUB
85% w/w ethyl alcohol with emollients
Indication: Significantly reduces the number
of micro-organisms on the hands and forearms
prior to surgery or patient care.
Fast-actng. Dermatologically tested.
Gentle to skin.
Read Drug Facts panel before use.
Lot:
Use by:
Questions? Call 1-800-MEDLINE
NDC 65616-008-06
1000 mL 33.8 fl.oz.



-
INGREDIENTS AND APPEARANCE
STERILLIUM RUB FRAGRANCE FREE
alcohol liquidProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:65616-008 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ALCOHOL (UNII: 3K9958V90M) (ALCOHOL - UNII:3K9958V90M) ALCOHOL 89.5 mL in 100 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) ISOPROPYL ALCOHOL (UNII: ND2M416302) MYRISTYL ALCOHOL (UNII: V42034O9PU) GLYCERIN (UNII: PDC6A3C0OX) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:65616-008-06 1000 mL in 1 BOTTLE, PUMP; Type 0: Not a Combination Product 07/31/2019 2 NDC:65616-008-08 50 mL in 1 BOTTLE, SPRAY; Type 0: Not a Combination Product 07/31/2019 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug 505G(a)(3) 07/31/2019 Labeler - BODE Chemie GmbH (316039007) Establishment Name Address ID/FEI Business Operations Kutol Products Company 004236139 manufacture(65616-008)




