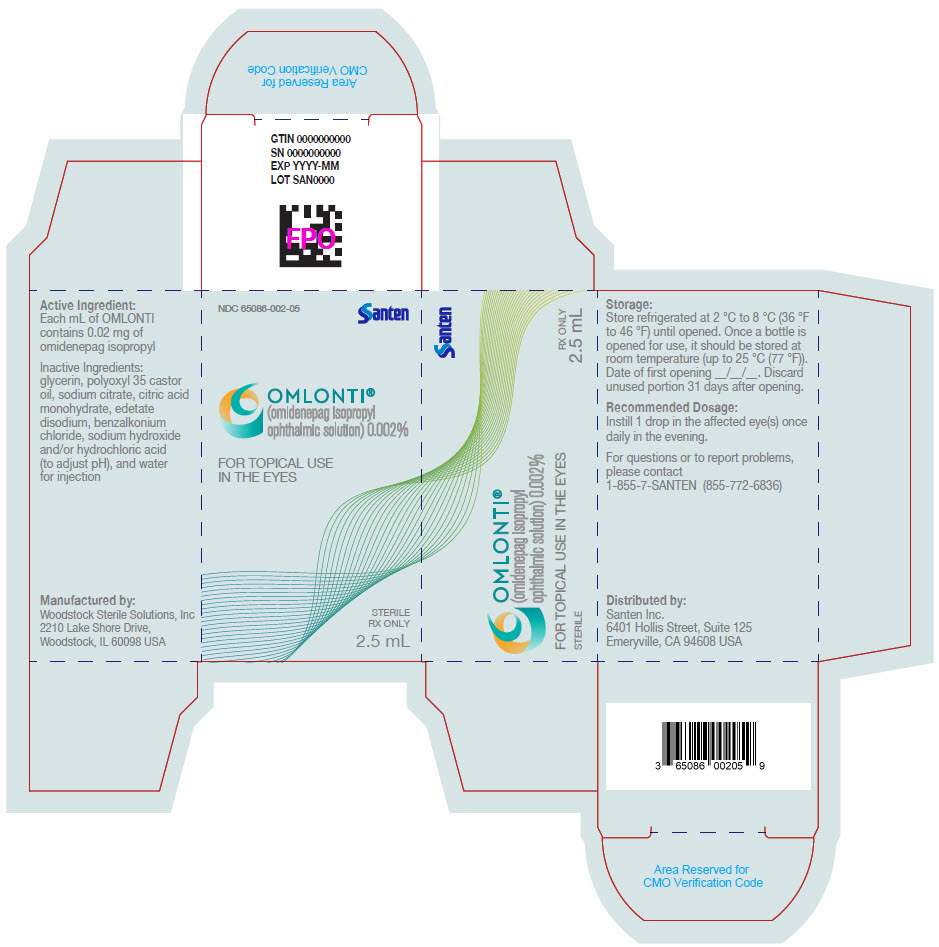
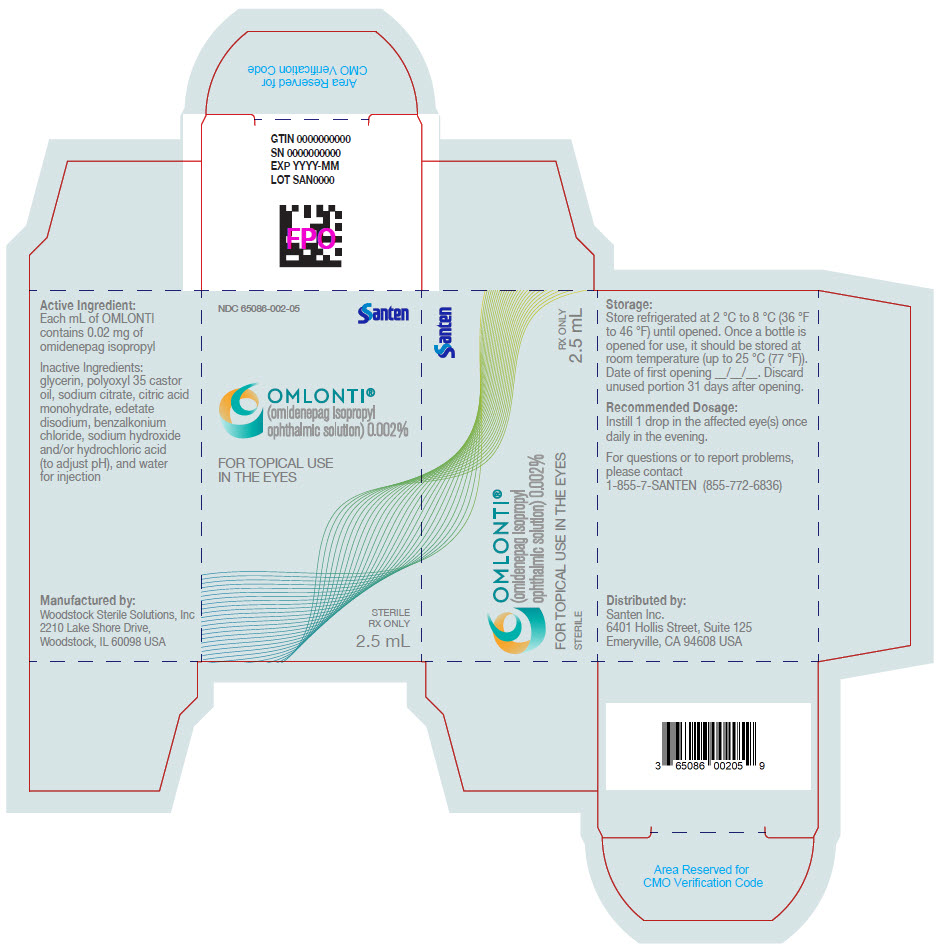
Label: OMLONTI- omidenepag isopropyl solution/ drops
-
Contains inactivated NDC Code(s)
NDC Code(s): 65086-002-01, 65086-002-05 - Packager: Santen Incorporated
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: New Drug Application
Drug Label Information
Updated October 9, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATION
These highlights do not include all the information needed to use OMLONTI ® safely and effectively. See full prescribing information for OMLONTI ®.
OMLONTI ® (omidenepag isopropyl ophthalmic solution) 0.002%, for topical ophthalmic use
Initial U.S. Approval: 2022INDICATIONS AND USAGE
Omlonti (omidenepag isopropyl ophthalmic solution) 0.002%, is a relatively selective prostaglandin E2 (EP2) receptor agonist, indicated for the reduction of elevated intraocular pressure (IOP) in patients with open-angle glaucoma or ocular hypertension. ( 1)
DOSAGE AND ADMINISTRATION
The recommended dosage is one drop in the affected eye(s) once daily in the evening. ( 2.1)
DOSAGE FORMS AND STRENGTHS
Ophthalmic solution containing 0.002% (0.02 mg/mL) of omidenepag isopropyl. ( 3)
CONTRAINDICATIONS
None ( 4)
WARNINGS AND PRECAUTIONS
ADVERSE REACTIONS
The most common adverse reactions with incidence ≥ 1% are conjunctival hyperemia (9%), photophobia (5%), vision blurred (4%), dry eye (3%), instillation site pain (3%), eye pain (2%), ocular hyperemia (2%), punctate keratitis (2%), headache (2%), eye irritation (1%), and visual impairment (1%). ( 6.1)
To report SUSPECTED ADVERSE REACTIONS, contact Santen at 1-855-7-SANTEN (855-772-6836) or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
See 17 for PATIENT COUNSELING INFORMATION.
Revised: 9/2022
-
Table of Contents
FULL PRESCRIBING INFORMATION: CONTENTS*
1 INDICATIONS AND USAGE
2 DOSAGE AND ADMINISTRATION
2.1 Recommended Dosage
2.2 Administration Instructions
3 DOSAGE FORMS AND STRENGTHS
4 CONTRAINDICATIONS
5 WARNINGS AND PRECAUTIONS
5.1 Pigmentation
5.2 Eyelash Changes
5.3 Ocular Inflammation
5.4 Macular Edema
5.5 Risk of Contamination and Potential Injury to the Eye
6 ADVERSE REACTIONS
6.1 Clinical Trials Experience
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
8.2 Lactation
8.3 Females and Males of Reproductive Potential
8.4 Pediatric Use
8.5 Geriatric Use
11 DESCRIPTION
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
12.3 Pharmacokinetics
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
13.2 Animal Toxicology and/or Pharmacology
14 CLINICAL STUDIES
16 HOW SUPPLIED/STORAGE AND HANDLING
17 PATIENT COUNSELING INFORMATION
- *
- Sections or subsections omitted from the full prescribing information are not listed.
- 1 INDICATIONS AND USAGE
-
2 DOSAGE AND ADMINISTRATION
2.1 Recommended Dosage
The recommended dosage is one drop in the affected eye(s) once daily in the evening.
2.2 Administration Instructions
Gently shake the bottle prior to administration. If more than one topical ophthalmic drug is being used, the drugs should be administered at least five (5) minutes apart. Contact lenses should be removed prior to the administration of Omlonti, and may be reinserted 15 minutes after administration [see Patient Counseling Information (17)].
- 3 DOSAGE FORMS AND STRENGTHS
- 4 CONTRAINDICATIONS
-
5 WARNINGS AND PRECAUTIONS
5.1 Pigmentation
Omlonti (omidenepag isopropyl ophthalmic solution) 0.002%, is a prodrug of omidenepag, a relatively selective EP2 receptor agonist. Pigmentation is expected to increase as long as omidenepag isopropyl ophthalmic solution is administered. The pigmentation change is due to increased melanin content in the melanocytes rather than to an increase in the number of melanocytes. After discontinuation of Omlonti, pigmentation of the iris is likely to be permanent, while pigmentation of the periorbital tissue and eyelash changes are likely to be reversible in most patients. Patients who receive prostaglandin analogs, including Omlonti, should be informed of the possibility of increased pigmentation, including permanent changes. The long-term effects of increased pigmentation are not known.
Iris color change may not be noticeable for several months to years. Typically, the brown pigmentation around the pupil spreads concentrically towards the periphery of the iris and the entire iris or parts of the iris become more brownish. Neither nevi nor freckles of the iris appear to be affected by treatment. While treatment with Omlonti (omidenepag isopropyl ophthalmic solution), 0.002% can be continued in patients who develop noticeably increased iris pigmentation, these patients should be examined regularly [see Patient Counseling Information (17)] .
5.2 Eyelash Changes
Omlonti may gradually change eyelashes and vellus hair in the treated eye. These changes include increased length, thickness, and the number of lashes or hairs. Eyelash changes are usually reversible upon discontinuation of treatment.
5.3 Ocular Inflammation
Ocular inflammation has been reported in patients taking Omlonti. Omlonti should be used with caution in patients with active ocular inflammation, including iritis/uveitis.
-
6 ADVERSE REACTIONS
The following clinically significant adverse reactions are described elsewhere in the labeling:
- Pigmentation [see Warnings and Precautions (5.1)]
- Eyelash Changes [see Warnings and Precautions (5.2)]
- Ocular Inflammation [see Warnings and Precautions (5.3)]
- Macular Edema [see Warnings and Precautions (5.4)]
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
The data described below reflect exposure to Omlonti in 600 patients for up to 3 months . The most common adverse reactions with incidence ≥ 1% are conjunctival hyperemia (9%), photophobia (5%), vision blurred (4%), dry eye (3%), instillation site pain (3%), eye pain (2%), ocular hyperemia (2%), punctate keratitis (2%), headache (2%), eye irritation (1%), and visual impairment (1%).
-
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Risk Summary
There are no available data on the use of Omlonti in pregnant women. In animal reproduction studies, subcutaneous administration of omidenepag isopropyl to pregnant rabbits throughout the period of organogenesis produced fetal skeletal anomalies at a dose of 24 times the clinical dose, based on estimated plasma C max. Omidenepag isopropyl was not teratogenic in rats when administered subcutaneously at 1 mg/kg/day, 2,452 times the clinical dose, based on estimated plasma C max (see Data) .
Data
Animal Data
An embryofetal development study was conducted in pregnant rabbits administered omidenepag isopropyl once daily by subcutaneous injection at 0.008, 0.08, or 0.8 mg/kg/day from gestation Day 6 to 18, a period which covers implantation and the period of organogenesis in rabbits. Fetal skeletal anomalies (thoracic misaligned centrum and hemivertebra, fused sternebra, absent rib) were observed at 0.008 mg/kg/day (24 times the maximum recommended human ocular dose [MRHOD], based on estimated plasma C max). Additional fetal skeletal anomalies were observed at 0.08 mg/kg (absent thoracic arch, fused rib) and 0.8 mg/kg (misaligned and misshapen cervical vertebrae), corresponding to 256 times and 3,696 times the MRHOD based on estimated plasma C max, respectively. Increases in preimplantation loss and post-implantation loss were observed at 0.8 mg/kg/day. The rabbit maternal, No Observed Adverse Effect Level [NOAEL] was 0.8 mg/kg/day.
An embryofetal development study was conducted in pregnant rats administered omidenepag isopropyl once daily by subcutaneous injection at 0.01, 0.1, or 1 mg/kg/day from gestation Day 6 to 17, to target the period of organogenesis. Omidenepag isopropyl was not found to have any effect on embryo-fetal development in rats at up to 1 mg/kg/day (2,452 times the MRHOD based on estimated plasma C max). The rat maternal NOAEL was 1 mg/kg/day. In a pre/postnatal development study, treatment of pregnant rats with omidenepag isopropyl subcutaneously from gestation day 6 to lactation day 20 at 0.01, 0.1, or 1 mg/kg/day resulted in no adverse effects. The NOAEL for pre- and postnatal development was 1 mg/kg/day (2,452 times the MRHOD based on estimated plasma C max).
8.2 Lactation
Risk Summary
There are no data on the presence of Omlonti in human milk, the effects on the breastfed infant, or the effects on milk production. However, systemic exposure to omidenepag following topical ocular administration is low [see Clinical Pharmacology (12.3)], and it is not known whether measurable levels of omidenepag would be present in maternal milk following topical ocular administration. The developmental and health benefits of breastfeeding should be considered along with the mother's clinical need for Omlonti and any unknown potential adverse effects on the breast-fed child from Omlonti.
8.3 Females and Males of Reproductive Potential
Infertility
There are no data on the effects of Omlonti on human fertility. No impairment of fertility has been reported in animals receiving omidenepag isopropyl subcutaneously at doses up to 2,452 times the clinical dose based on estimated plasma C max [see Nonclinical Toxicology (13.1)] .
-
11 DESCRIPTION
Omlonti (omidenepag isopropyl ophthalmic solution) 0.002%, contains the prodrug form of the active omidenepag, a relatively selective prostaglandin EP2 receptor agonist with ocular hypotensive activity. Omidenepag isopropyl is a white to light brown crystal or crystalline powder and practically insoluble in water. Omidenepag isopropyl's chemical name is Glycine, N-[6-[[[[4-(1H-pyrazol-1-yl)phenyl]methyl](3-pyridinylsulfonyl)amino]methyl]-2-pyridinyl]-, 1-methylethyl ester and has the following structure:
Structural Formula
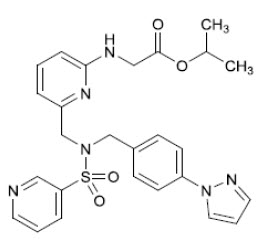
Formula of the free base: C 26H 28N 6O 4S. Molecular weight: 520.61
Omlonti appears as a clear, colorless solution. It is supplied as a sterile, isotonic, buffered aqueous solution of omidenepag isopropyl with a target pH of 5.8 and an osmolality of approximately 285 mOsmol/kg.
Each mL of Omlonti contains: Active: 0.02 mg of omidenepag isopropyl.
Preservative: 0.005% benzalkonium chloride.
Inactive ingredients: glycerin, polyoxyl 35 castor oil, sodium citrate, citric acid monohydrate, edetate disodium, sodium hydroxide and/or hydrochloric acid (to adjust pH), and water for injection.
-
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
Omidenepag is a relatively selective EP2 receptor agonist which decreases intraocular pressure (IOP). The exact mechanism of action is unknown at this time. Elevated IOP represents a major risk factor for glaucomatous field loss. The higher the level of IOP, the greater the likelihood of optic nerve damage and visual field loss.
12.3 Pharmacokinetics
Absorption
Omlonti is absorbed through the cornea where prodrug omidenepag isopropyl is hydrolyzed to become biologically active metabolite, omidenepag. After once daily ocular administration of one drop of omidenepag isopropyl 0.0025% eye drops to both eyes in humans for 7 days, plasma concentrations of omidenepag reached C max at 10-15 minutes. Systemic exposure was similar between days 1 and 7, indicating no systemic accumulation.
Elimination
Metabolism
After topical ocular administration, omidenepag isopropyl is rapidly metabolized in the eye to omidenepag (active moiety) by carboxylesterase-1. The pharmacologically active form, omidenepag is further metabolized by liver through oxidation, N-dealkylation, glucuronidation, sulfate conjugation or taurine conjugation.
Excretion
In rats, 0.03% 14C-Omlonti was instilled in both eyes as a single dose (5 mcL/eye, 3 mcg/animal). By 168 hours after ocular instillation, 89% of the administered radioactive dose had been excreted. Specifically, 83% and 4% of the administered radioactive dose were excreted in the feces and urine, respectively, and radioactivity in expired air was below the lower limit of quantitation.
-
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
Carcinogenesis
Lifetime rodent studies have not been performed to evaluate the carcinogenic potential of omidenepag isopropyl or omidenepag.
In rats dosed by subcutaneous injection daily for 26 weeks, nephroblastoma and a spermatic cord tumor were found at 0.003 mg/kg/day (33 times the RHOD based on the estimated area under the curve [AUC]). Mammary adenocarcinoma and pituitary pars distalis adenomas were observed at 0.03 mg/kg/day (319 fold the RHOD based on the estimated AUC).
Mutagenesis
Omidenepag was not mutagenic in the bacterial reverse mutation (Ames) test and the in vivo mouse micronucleus test. Omidenepag isopropyl was positive (mutagenic and clastogenic) without metabolic activation in the in vitro mouse lymphoma forward mutation assay. [see Clinical Pharmacology (12.3)] .
Impairment of Fertility
In a rat fertility and early embryonic development study, daily subcutaneous doses of omidenepag isopropyl did not affect male or female fertility at doses up to 1 mg/kg/day (2,452 times the MRHOD based on estimated plasma C max).
The rabbit embryofetal development study administered omidenepag isopropyl to pregnant rabbits beginning on gestation day 6, covering the period of implantation [see Use in Specific Populations (8.1)] . Preimplantation losses were observed at 0.8 mg/kg/day (3,696 times the MRHOD based on estimated plasma C max). The NOAEL for rabbit preimplantation loss was 0.08 mg/kg/day (256 times the MRHOD based on estimated plasma C max) [see Use in Specific Populations (8.1)] .
13.2 Animal Toxicology and/or Pharmacology
Nasal cavity respiratory epithelium metaplasia was observed in cynomolgus monkeys receiving unilateral topical ocular instillations of omidenepag isopropyl at 0.003% (0.9 mcg/eye) [1.07 fold the MRHOD, based on ocular dose comparison]. In a 13-week monkey study, the 0.9 mcg/eye dose was associated with nasal cavity respiratory epithelial metaplasia, and increased nasal cavity goblet cell respiratory epithelium mucosa. In a 39-week monkey study, the 0.9 mcg/eye dose was associated with increased incidence and severity of nasal cavity respiratory epithelium metaplasia. These findings were present in both the treated side and untreated contralateral side, and they were also observed in the vehicle group [see Adverse Reactions (6)] .
-
14 CLINICAL STUDIES
Omlonti was evaluated in three randomized and controlled clinical trials in subjects with open-angle glaucoma or ocular hypertension with average baseline IOP of 24-26 mm Hg. The double-masked treatment duration was 3 months in all 3 studies. The third study included a 9-month open-label treatment period following the 3-month double-masked treatment period.
In the three studies, IOP reductions were observed for all treatment arms. In the Omlonti arm, the reduction in IOP ranged from 5-7 mm Hg across all three studies. The corresponding reductions for the timolol and latanoprost arms were 5-7 mm Hg and 6-8 mm Hg, respectively.
-
16 HOW SUPPLIED/STORAGE AND HANDLING
Omlonti (omidenepag isopropyl ophthalmic solution) 0.002%, is supplied as a 2.5 mL sterile solution in 5 mL white low density polyethylene bottles with linear low density polyethylene dropper tips, high density polyethylene screw caps and tamper-evident low density polyethylene overcaps.
NDC 65086-002-05
-
17 PATIENT COUNSELING INFORMATION
Advise the patient to read the FDA-approved patient labeling (Instructions for Use).
Potential for Pigmentation
Patients should be advised about the potential for increased brown pigmentation of the iris, which may be permanent. Patients should also be informed about the possibility of eyelid skin darkening, which is usually reversible after discontinuation of Omlonti.
Potential for Eyelash Changes
Patients should also be informed of the possibility of eyelash and vellus hair changes in the treated eye during treatment with Omlonti. These changes may result in a disparity between eyes in length, thickness, pigmentation, number of eyelashes or vellus hairs, and/or direction of eyelash growth. Eyelash changes are usually reversible upon discontinuation of treatment.
Handling the Container
Advise patients to avoid touching the tip of the bottle to the eye or any surface, as this may contaminate the solution. Advise patients to not touch the tip to their eye to avoid the potential for injury to the eye. The product should be refrigerated before opening, and once it is opened, it can be stored at room temperature for up to 31 days . [see How Supplied/Storage and Handling (16)] .
When to Seek Physician Advice
Advise patients that if they develop a new ocular condition (e.g., trauma, infection, or inflammation), experience a sudden decrease in visual acuity, have ocular surgery, or develop any ocular reactions, particularly conjunctivitis and eyelid reactions, they should immediately seek their physician's advice concerning the continued use of Omlonti.
Use with Contact Lenses
Contact lenses should be removed prior to administration of the solution. Lenses may be reinserted 15 minutes after administration.
Use with Other Ophthalmic Drugs
Advise patients that if more than one topical ophthalmic drug is being used, the drugs should be administered at least five (5) minutes apart [see Dosage and Administration (2)] .
- SPL UNCLASSIFIED SECTION
-
INSTRUCTIONS FOR USE
OMLONTI® [om lon'tee]
(omidenepag isopropyl ophthalmic solution) 0.002%
for topical ophthalmic useRead this Instructions for Use before you start using Omlonti and each time you get refills. There may be new and updated information. This leaflet does not take the place of talking to your healthcare provider about your medical condition or your treatment.
Parts of your Omlonti multi-dose bottle 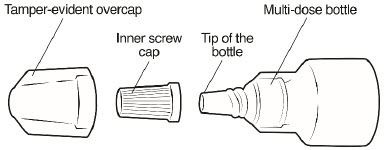
Important information you need to know before using Omlonti
- Omlonti is for use in the eye.
- Use Omlonti exactly as your healthcare provider tells you to use it.
- After you remove the Omlonti multi-dose bottle from the carton, make sure the tamper-evident overcap is not broken. If the tamper-evident overcap is broken, Omlonti may not be safe for you to use.
- If you use Omlonti with other eye medicines, you should wait at least 5 minutes between using Omlonti and the other medicines.
- If you wear contact lenses, remove your contact lenses before using Omlonti. You should wait 15 minutes after using Omlonti before placing your contact lenses back in your eye.
- Call your healthcare provider right away if you develop new eye problems including: an eye injury, eye infection, eye pain, light sensitivity, blurry vision, decreased vision, swelling and redness of the eye, problems with your eyelids, changes in the appearance of your eyes, headache, or eye surgery.
- If you develop blurred vision or light sensitivity after using Omlonti, do not drive or operate heavy machinery until the symptoms disappear.
- Omlonti can cause color changes in your iris and eyelid and may change the length and color of your eyelashes.
Using Omlonti
Follow Step 1 to Step 11 each time you use Omlonti. Ask your healthcare provider for help if you do not understand how to use Omlonti.
Step 1. Wash your hands thoroughly with soap and water. If you cannot wash your hands, you can clean your hands using hand sanitizer. 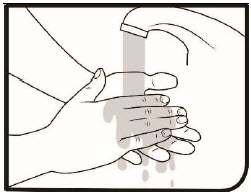
Step 2. Turn the tamper-evident overcap to the left to open the multi-dose bottle. 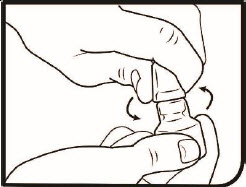
Step 3. Remove the tamper-evident overcap and throw it away. 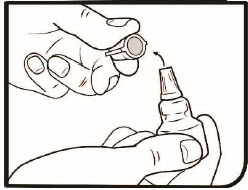
Step 4. Gently shake the bottle. 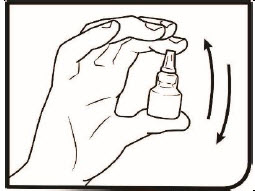
Step 5. Turn the inner screw cap to the left to remove it. Do not throw the inner screw cap away. 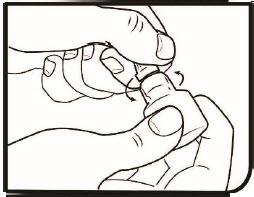
Step 6. Pull down your lower eyelid with a clean finger. The medicine will go into the space between your lower eyelid and your eye. 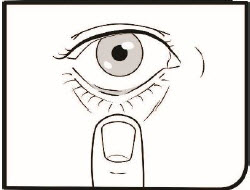
Step 7. Tilt your head back and look up. 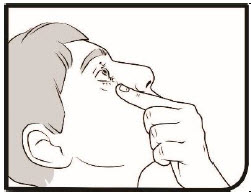
Step 8. Gently squeeze the multi-dose bottle to release 1 drop of the medicine in your eye. Do not let the tip of the multi-dose bottle touch your eye or any other surfaces. If a drop misses your eye, try again. 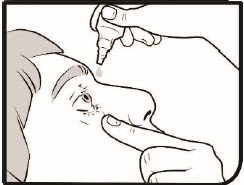
Step 9. Blink a few times so that the medicine spreads across your eye. 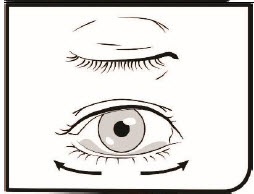
Step 10. After using Omlonti, gently close your eyelid and press the corner of your eye closest to your nose for 2 minutes. 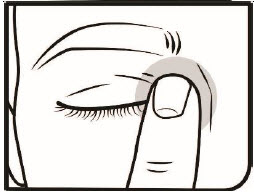
Step 11. Repeat Step 6 to Step 10 for your other eye if you need to use drops in both eyes. Storing Omlonti
- Store unopened Omlonti in the refrigerator between 36°F to 46°F (2°C to 8°C).
- After opening, store Omlonti up to 31 days at room temperature between 68°F to 86°F (20° to 30°C).
- Do not use Omlonti if the expiration date (EXP YYYY-MM) on the outer carton and multi-dose bottle have passed. The expiration date is the last day of that month.
- After removing the tamper-evident overcap, keep the multi-dose bottle closed with the inner screw cap on to avoid contamination.
- Keep Omlonti and all medicines out of the reach of children.
Additional Information
- If you have questions about Omlonti, talk to your healthcare provider, go to the Santen's website (https://www.santen.com/), or call Santen Medical Information: 855-7-SANTEN (855-772-6836).
Distributed by:
Santen Incorporated
6401 Hollis St., Suite 125
Emeryville, CA 94608
This Instructions for Use has been approved by the U.S. Food and Drug Administration.
Approved: 9/2022 - PRINCIPAL DISPLAY PANEL - 2.5 mL Bottle Carton
-
INGREDIENTS AND APPEARANCE
OMLONTI
omidenepag isopropyl solution/ dropsProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:65086-002 Route of Administration OPHTHALMIC Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength OMIDENEPAG ISOPROPYL (UNII: G0G0H52U6K) (OMIDENEPAG - UNII:Z95F9F9LU4) OMIDENEPAG ISOPROPYL 0.02 mg in 1 mL Inactive Ingredients Ingredient Name Strength TRISODIUM CITRATE DIHYDRATE (UNII: B22547B95K) CITRIC ACID MONOHYDRATE (UNII: 2968PHW8QP) POLYOXYL 35 CASTOR OIL (UNII: 6D4M1DAL6O) BENZALKONIUM CHLORIDE (UNII: F5UM2KM3W7) EDETATE DISODIUM (UNII: 7FLD91C86K) GLYCERIN (UNII: PDC6A3C0OX) SODIUM HYDROXIDE (UNII: 55X04QC32I) HYDROCHLORIC ACID (UNII: QTT17582CB) WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:65086-002-05 1 in 1 CARTON 09/23/2022 1 2.5 mL in 1 BOTTLE, PLASTIC; Type 2: Prefilled Drug Delivery Device/System (syringe, patch, etc.) 2 NDC:65086-002-01 1 in 1 CARTON 09/23/2022 2 2.5 mL in 1 BOTTLE, PLASTIC; Type 2: Prefilled Drug Delivery Device/System (syringe, patch, etc.) Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA215092 09/23/2022 Labeler - Santen Incorporated (869321331)