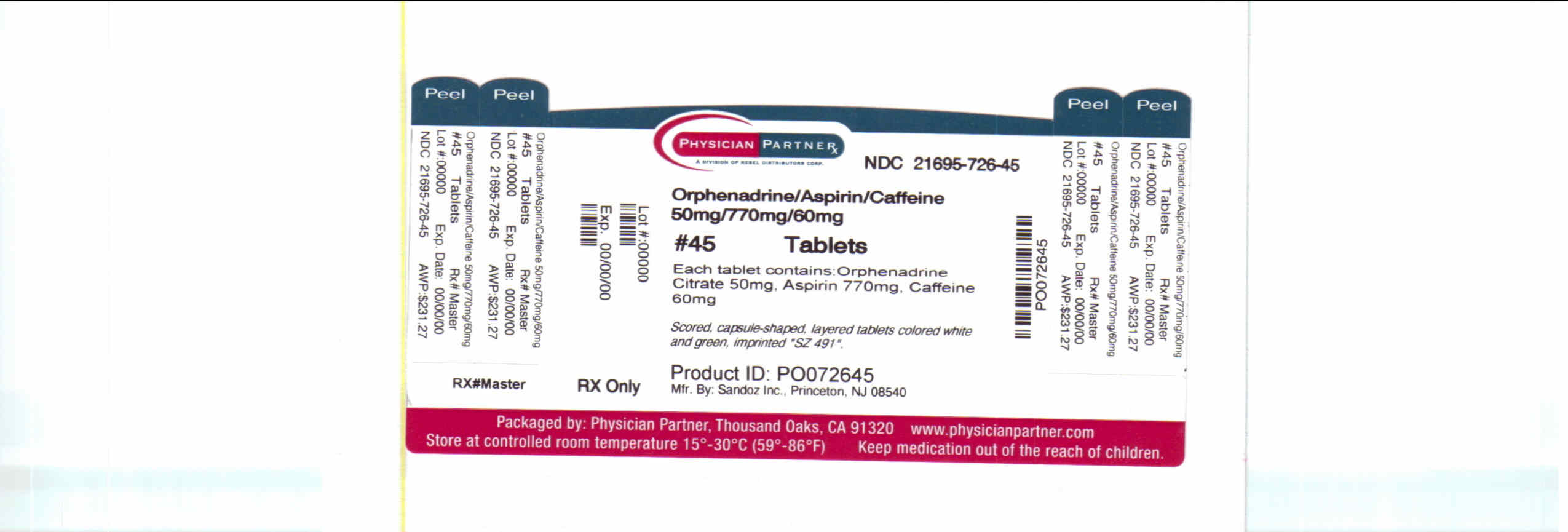
Label: ORPHENADRINE CITRATE, ASPIRIN AND CAFFEINE tablet, multilayer
-
Contains inactivated NDC Code(s)
NDC Code(s): 21695-726-30, 21695-726-45 - Packager: Rebel Distributors Corp
- This is a repackaged label.
- Source NDC Code(s): 0185-0714
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated October 22, 2010
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
DESCRIPTION
Each tablet, for oral administration, contains Orphenadrine Citrate USP, 25 mg or 50 mg, Aspirin USP, 385 mg or 770 mg, Caffeine USP, 30 mg or 60 mg.
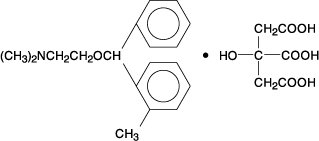
Orphenadrine citrate, N, N-Dimethyl-2-[(o-methyl-α-phenylbenzyl)oxy]ethylamine citrate (1:1), is a centrally acting (brain stem) compound. It occurs as a white, practically odorless, crystalline powder, having a bitter taste. Its molecular formula is C18H23NO.C6H8O7 with a molecular weight of 461.51. The structural formula is shown below.
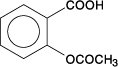
Aspirin, salicylic acid acetate, is a non-opiate analgesic, anti-inflammatory and antipyretic agent. It occurs as a white, crystalline tabular or needle like powder and is odorless or has a faint odor. Its molecular formula is C9H8O4, with a molecular weight of 180.16. The structural formula is shown below.
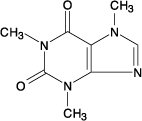
Caffeine, 1,3,7-trimethylxanthine, is a central nervous system stimulant which occurs as a white powder or white glistening needles. It also has a bitter taste. Its molecular formula is C8H10N4O2, with a molecular weight of 194.19. The structural formula is shown below.
Each tablet contains the following inactive ingredients: colloidal silicon dioxide, corn starch, croscarmellose sodium, FD&C Blue No. 1, FD&C Yellow No. 10, anhydrous lactose, microcrystalline cellulose, povidone, pregelatinized starch, stearic acid, and sodium lauryl sulfate.
-
CLINICAL PHARMACOLOGY
Orphenadrine citrate is a centrally acting (brain stem) compound which in animals selectively blocks facilitatory functions of the reticular formulation. Orphenadrine does not produce myoneural block, nor does it affect crossed extensor reflexes. Orphenadrine prevents nicotine-induced convulsions but not those produced by strychnine.
Chronic administration of Orphenadrine Citrate, Aspirin, and Caffeine to dogs and rats has revealed no drug-related toxicity. No blood or urine changes were observed, nor were there any macroscopic or microscopic pathological changes detected. Extensive experience with combinations containing aspirin and caffeine has established them as safe agents. The addition of orphenadrine citrate does not alter the toxicity of aspirin and caffeine.
The mode of therapeutic action of orphenadrine has not been clearly identified, but may be related to its analgesic properties. Orphenadrine citrate also possesses anticholinergic actions.
-
INDICATIONS AND USAGE
- Symptomatic relief of mild to moderate pain of acute musculoskeletal disorders.
- The orphenadrine component is indicated as an adjunct to rest, physical therapy, and other measures for the relief of discomfort associated with acute painful musculoskeletal conditions.
The mode of action of orphenadrine has not been clearly identified, but may be related to its analgesic properties. Orphenadrine Citrate, Aspirin, and Caffeine Tablets do not directly relax tense skeletal muscles in man.
-
CONTRAINDICATIONS
Because of the mild anti-cholinergic effect of orphenadrine, Orphenadrine Citrate, Aspirin, and Caffeine Tablets should not be used in patients with glaucoma, pyloric or duodenal obstruction, achalasia, prostatic hypertrophy, or obstructions at the bladder neck. Orphenadrine Citrate, Aspirin, and Caffeine Tablets are also contraindicated in patients with myasthenia gravis and in patients known to be sensitive to aspirin or caffeine.
The drug is contraindicated in patients who have demonstrated a previous hypersensitivity to the drug.
-
WARNINGS
Reye’s Syndrome may develop in individuals who have chicken pox, influenza, or flu symptoms. Some studies suggest possible association between the development of Reye’s Syndrome and the use of medicines containing salicylate or aspirin. Orphenadrine Citrate, Aspirin, and Caffeine Tablets contain aspirin and therefore are not recommended for use in patients with chicken pox, influenza, or flu symptoms. Orphenadrine Citrate, Aspirin, and Caffeine Tablets may impair the ability of the patient to engage in potentially hazardous activities such as operating machinery or driving a motor vehicle: ambulatory patients should therefore be cautioned accordingly.
Aspirin should be used with extreme caution in the presence of peptic ulcers and coagulation abnormalities.
-
PRECAUTIONS
Confusion, anxiety and tremors have been reported in a few patients receiving propoxyphene and orphenadrine concomitantly. As these symptoms may be simply due to an additive effect, reduction of dosage and/or discontinuation of one or both agents is recommended in such cases.
Safety of continuous long term therapy with Orphenadrine Citrate, Aspirin, and Caffeine Tablets has not been established; therefore, if Orphenadrine Citrate, Aspirin, and Caffeine Tablets are prescribed for prolonged use, periodic monitoring of blood, urine and liver function values is recommended.
-
ADVERSE REACTIONS
Side effects of Orphenadrine Citrate, Aspirin, and Caffeine Tablets are those seen with aspirin and caffeine or those usually associated with mild anticholinergic agents. These may include tachycardia, palpitation, urinary hesitancy or retention, dry mouth, blurred vision, dilation of the pupil, increased intraocular tension, weakness, nausea, vomiting, headache, dizziness, constipation, drowsiness, and rarely, urticaria and other dermatoses. Infrequently, an elderly patient may experience some degree of confusion. Mild central excitation and occasional hallucinations may be observed. These mild side effects can usually be eliminated by reduction in dosage. One case of aplastic anemia associated with the use of Orphenadrine Citrate, Aspirin, and Caffeine Tablets has been reported. No causal relationship has been established. Rare G.I. hemorrhage due to aspirin content may be associated with the administration of Orphenadrine Citrate, Aspirin, and Caffeine Tablets. Some patients may experience transient episodes of lightheadedness, dizziness or syncope.
- DOSAGE AND ADMINISTRATION
- HOW SUPPLIED
- SPL UNCLASSIFIED SECTION
- 50 mg/770 mg/60 mg Label
-
INGREDIENTS AND APPEARANCE
ORPHENADRINE CITRATE, ASPIRIN AND CAFFEINE
orphenadrine citrate, aspirin and caffeine tablet, multilayerProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:21695-726(NDC:0185-0714) Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ORPHENADRINE CITRATE (UNII: X0A40N8I4S) (ORPHENADRINE - UNII:AL805O9OG9) ORPHENADRINE CITRATE 50 mg ASPIRIN (UNII: R16CO5Y76E) (ASPIRIN - UNII:R16CO5Y76E) ASPIRIN 770 mg CAFFEINE (UNII: 3G6A5W338E) (CAFFEINE - UNII:3G6A5W338E) CAFFEINE 60 mg Inactive Ingredients Ingredient Name Strength ANHYDROUS LACTOSE (UNII: 3SY5LH9PMK) CROSCARMELLOSE SODIUM (UNII: M28OL1HH48) FD&C BLUE NO. 1 (UNII: H3R47K3TBD) D&C YELLOW NO. 10 (UNII: 35SW5USQ3G) CELLULOSE, MICROCRYSTALLINE (UNII: OP1R32D61U) POVIDONE (UNII: FZ989GH94E) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) SODIUM LAURYL SULFATE (UNII: 368GB5141J) STARCH, CORN (UNII: O8232NY3SJ) STEARIC ACID (UNII: 4ELV7Z65AP) Product Characteristics Color WHITE, GREEN Score no score Shape CAPSULE Size 2mm Flavor Imprint Code SZ491 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:21695-726-30 30 in 1 BOTTLE 2 NDC:21695-726-45 45 in 1 BOTTLE Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA074654 12/31/1996 Labeler - Rebel Distributors Corp (118802834) Establishment Name Address ID/FEI Business Operations Rebel Distributors Corp 118802834 RELABEL, REPACK