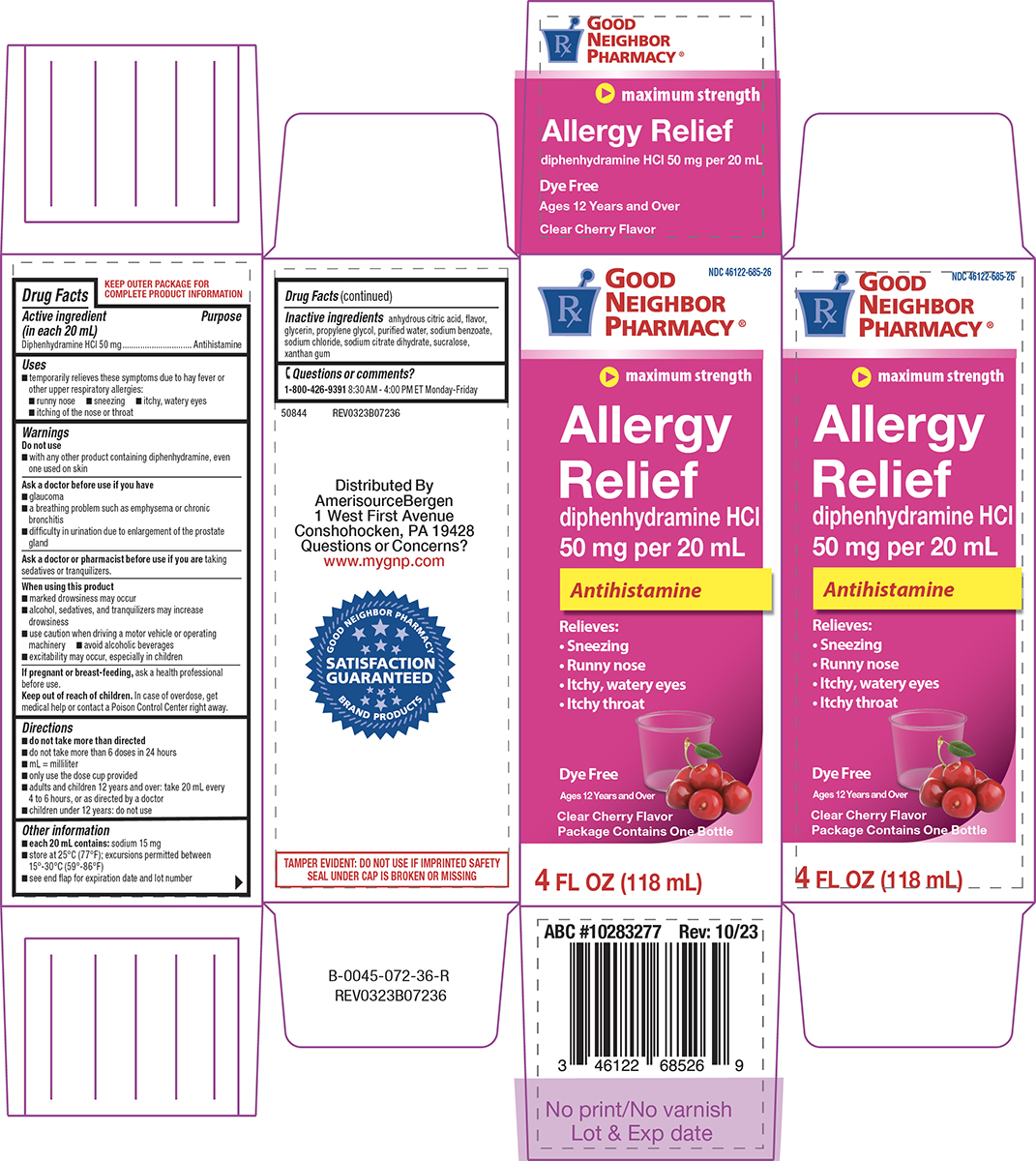
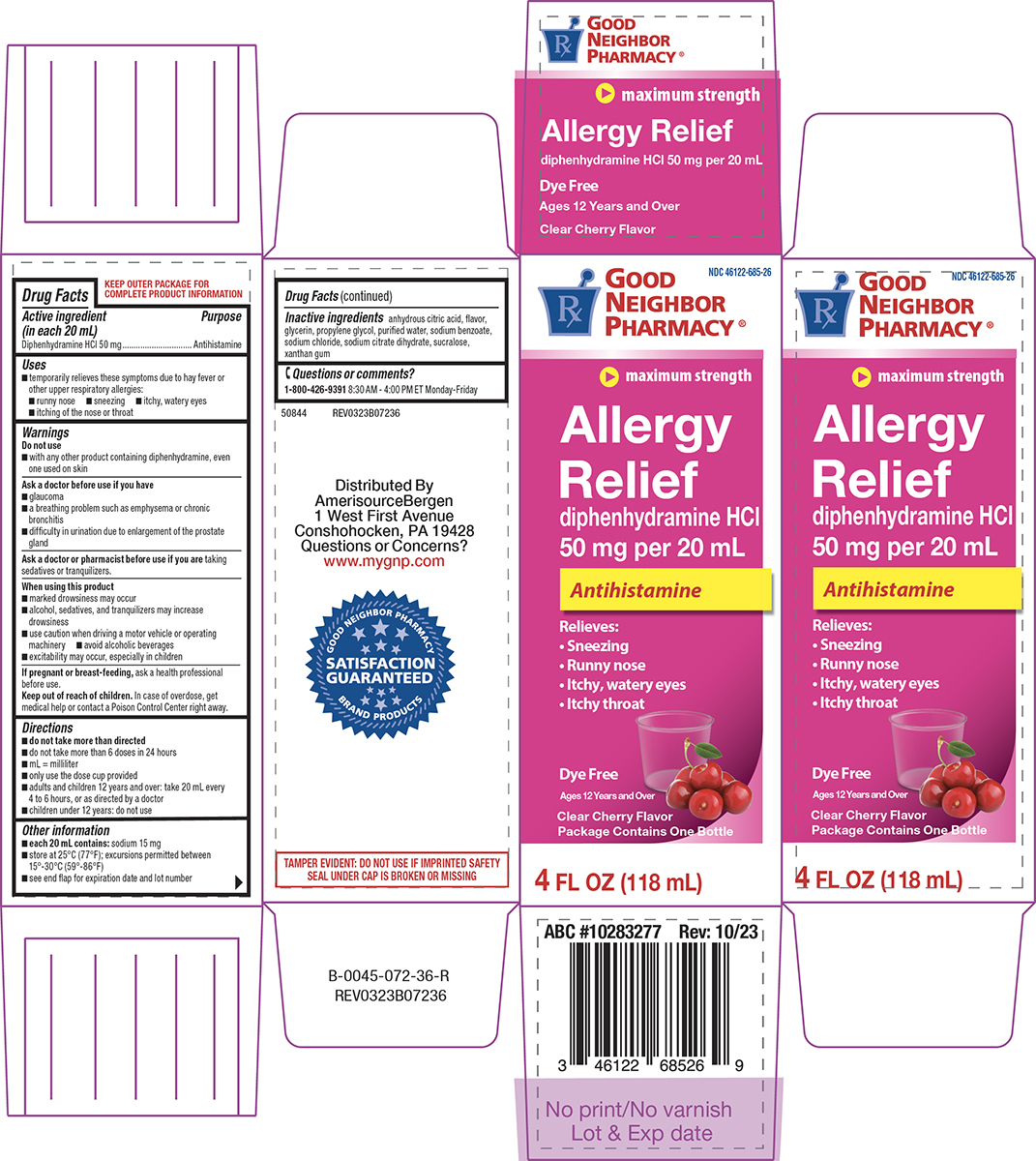
Label: ALLERGY RELIEF- diphenhydramine hcl solution
- NDC Code(s): 46122-685-26
- Packager: Amerisource Bergen
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated January 23, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Active ingredient (in each 20 mL)
- Purpose
- Uses
-
Warnings
Ask a doctor before use if you have
- glaucoma
- a breathing problem such as emphysema or chronic bronchitis
- difficulty in urination due to enlargement of the prostate gland
- Directions
- Other information
- Inactive ingredients
- Questions or comments?
-
Principal display panel
NDC 46122-685-26
GOOD
NEIGHBOR
PHARMACY®
maximum strength
Allergy
Relief
diphenhydramine HCl
50 mg per 20 mL
AntihistamineRelieves:
• Sneezing
• Runny nose
• Itchy, watery eyes
• Itchy throatDye Free
Clear Cherry Flavor
Package Contains One Bottle4 FL OZ (118 mL)
TAMPER EVIDENT: DO NOT USE IF IMPRINTED SAFETY
SEAL UNDER CAP IS BROKEN OR MISSING50844 REV0323B07236
Distributed By
AmerisourceBergen
1 West First Avenue
Conshohocken, PA 19428
Questions or Concerns?
www.mygnp.comABC #10283277 Rev: 10/23
Good Neighbor 44-072
-
INGREDIENTS AND APPEARANCE
ALLERGY RELIEF
diphenhydramine hcl solutionProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:46122-685 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength DIPHENHYDRAMINE HYDROCHLORIDE (UNII: TC2D6JAD40) (DIPHENHYDRAMINE - UNII:8GTS82S83M) DIPHENHYDRAMINE HYDROCHLORIDE 50 mg in 20 mL Inactive Ingredients Ingredient Name Strength ANHYDROUS CITRIC ACID (UNII: XF417D3PSL) GLYCERIN (UNII: PDC6A3C0OX) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) WATER (UNII: 059QF0KO0R) SODIUM BENZOATE (UNII: OJ245FE5EU) SODIUM CHLORIDE (UNII: 451W47IQ8X) TRISODIUM CITRATE DIHYDRATE (UNII: B22547B95K) SUCRALOSE (UNII: 96K6UQ3ZD4) XANTHAN GUM (UNII: TTV12P4NEE) Product Characteristics Color Score Shape Size Flavor CHERRY Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:46122-685-26 1 in 1 CARTON 01/26/2021 1 118 mL in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M012 01/26/2021 Labeler - Amerisource Bergen (007914906) Establishment Name Address ID/FEI Business Operations LNK International, Inc. 967626305 manufacture(46122-685) , pack(46122-685)