Label: ANTI-AGING EMULSION SPF 30- avobenzone, octinoxate, octisalate, oxybenzone cream
-
Contains inactivated NDC Code(s)
NDC Code(s): 68807-318-11 - Packager: Temmentec AG
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: OTC monograph final
DISCLAIMER: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
Drug Label Information
Updated January 3, 2017
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
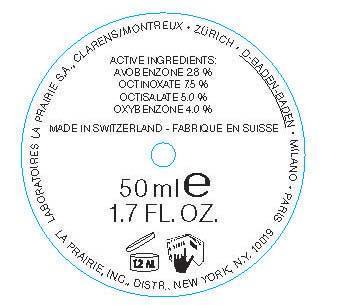
ACTIVE INGREDIENT
Active Ingredients Purpose
Avobenzone 2.7% Sunscreen
Octinoxate 7.5% Sunscreen
Octisalate 5.0% Sunscreen
Oxybenzone 4.0% Sunscreen
-helps prevent sunburn
-for skin that burns easily
-provides moderate protection against sunburn
-higher SPF gives more sunburn protection
-When using this product keep out of eyes
-Rinse with water to remove
-Stop use and ask a doctor if rash or irritation develops and lasts
-Do not use on infants under 6 months.
-apply daily after cleansing and toning
-smooth over face and throat
-apply evenly before sun exposure
-reapply as needed or after towel drying, swimming or perspiring
Inactive Ingredients: Water (Aqua), Butylene Glycol, Isostearyl Neopentanoate, Glycerin, Coco-Glucoside, Cyclohexasiloxane, Ethoxydiglycol, PEG-100 Stearate, Isononyl Isononanoate, Glyceryl Stearate, Glycoproteins, Panax Ginseng Root Extract, Equisetum Arvense (Horsetail) Extract, Olea Europaea (Olive) Oil Unsaponifiables, Ursolic Acid, IIomastat, Algin, Caprylyl/Capric Triglyceride, Coconut Alcohol, Caprylyl/Capryl Glucoside, Acacia Senegal Gum, Ascorbyl Tetraisopalmitate, Serine, Tocopherol, DNA, Triticum Vulgare (Wheat) Germ Oil Unsaponifiables, Carnosine, Camellia Sinensis Leaf Extract, Glycine Soja (Soybean) Oil Unsaponifiables, PEG-8, Phospholipids, Plankton Extract, Cyclopentasiloxane, Isodecyl Salicylate, Isopropylbenzyl Salicylate, Pollen Extract, Hydrolyzed Malt Extract, Propylene Glycol, Oligopeptide-5, Angelica Kelskei Extract, Oligopeptide-4, Dimethicone, Lecithin, Silybum Marlanum (Lady's Thistle) Fruit Extract, Tocopheryl Linoleate/Oleate, Echium Plantagineum Seed Oil, Polymethyl Methacrylate, Lauroyl Lysine, Sodium Acrylate/Acryloyldimethyl Taurate Copolymer, Sucrose, Xanthan Gum, Disodium EDTA, Alcohol, Acrylates/C10-30 Alkyl Acrylate Crosspolymer, Polyisobutene, Carbomer, PPG-2 Isoceteth-20 Acetate, Mica, Fragrance (Parfum), Benzyl Alcohol, Linalool, Hydroxycitronellal, Alpha-Isomethyl Ionone, Amyl Cinnamal, Hexyl Cinnamal, Evernia Furfuracea (Treemoss) Extract, Geraniol, Benzyl Benzoate, Butylphenyl Methylpropional, Citronellol, Eugenol, Benzyl Salicylate, Limonene, Phenoxyethanol, Potassium Sorbate, Chlorophenesin, Methylparaben, Benzoic Acid, Ethylparaben.

- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
ANTI-AGING EMULSION SPF 30
avobenzone, octinoxate, octisalate, oxybenzone creamProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:68807-318 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Avobenzone (UNII: G63QQF2NOX) (Avobenzone - UNII:G63QQF2NOX) Avobenzone 2.8 g in 100 kg Octinoxate (UNII: 4Y5P7MUD51) (Octinoxate - UNII:4Y5P7MUD51) Octinoxate 7.5 g in 100 kg Octisalate (UNII: 4X49Y0596W) (Octisalate - UNII:4X49Y0596W) Octisalate 5 g in 100 kg OXYBENZONE (UNII: 95OOS7VE0Y) (OXYBENZONE - UNII:95OOS7VE0Y) OXYBENZONE 4 g in 100 kg Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) BUTYLENE GLYCOL (UNII: 3XUS85K0RA) GLYCERIN (UNII: PDC6A3C0OX) COCO GLUCOSIDE (UNII: ICS790225B) CYCLOMETHICONE 6 (UNII: XHK3U310BA) DIETHYLENE GLYCOL MONOETHYL ETHER (UNII: A1A1I8X02B) PEG-100 STEARATE (UNII: YD01N1999R) GLYCERYL MONOSTEARATE (UNII: 230OU9XXE4) PANAX GINSENG ROOT WATER (UNII: P9T4K47OM0) EQUISETUM ARVENSE TOP (UNII: 1DP6Y6B65Z) URSOLIC ACID (UNII: P3M2575F3F) CAPRYLIC/CAPRIC ACID (UNII: DI775RT244) ACACIA (UNII: 5C5403N26O) SERINE (UNII: 452VLY9402) CARNOSINE (UNII: 8HO6PVN24W) BANCHA TEA LEAF/TWIG (UNII: EWI42IEH1C) SOYBEAN OIL (UNII: 241ATL177A) POLYETHYLENE GLYCOL 400 (UNII: B697894SGQ) CYCLOMETHICONE 5 (UNII: 0THT5PCI0R) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) DIMETHICONE (UNII: 92RU3N3Y1O) MILK THISTLE (UNII: U946SH95EE) ECHIUM PLANTAGINEUM SEED OIL (UNII: PIB7XBU8XW) SUCROSE (UNII: C151H8M554) XANTHAN GUM (UNII: TTV12P4NEE) CARBOMER INTERPOLYMER TYPE A (ALLYL SUCROSE CROSSLINKED) (UNII: 59TL3WG5CO) POLYISOBUTYLENE (35000 MW) (UNII: 98553S1MHQ) CARBOMER 934 (UNII: Z135WT9208) MICA (UNII: V8A1AW0880) BENZYL ALCOHOL (UNII: LKG8494WBH) LINALOOL, (+/-)- (UNII: D81QY6I88E) HYDROXYCITRONELLAL (UNII: 8SQ0VA4YUR) .ALPHA.-AMYLCINNAMALDEHYDE (UNII: WC51CA3418) GERANIOL (UNII: L837108USY) BENZYL BENZOATE (UNII: N863NB338G) BUTYLPHENYL METHYLPROPIONAL (UNII: T7540GJV69) CITRONELLAL (UNII: QB99VZZ7GZ) EUGENOL (UNII: 3T8H1794QW) BENZYL SALICYLATE (UNII: WAO5MNK9TU) LIMONENE, (+)- (UNII: GFD7C86Q1W) PHENOXYETHANOL (UNII: HIE492ZZ3T) POTASSIUM SORBATE (UNII: 1VPU26JZZ4) CHLORPHENESIN (UNII: I670DAL4SZ) METHYLPARABEN (UNII: A2I8C7HI9T) ETHYLPARABEN (UNII: 14255EXE39) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:68807-318-11 78.4 kg in 1 DRUM; Type 0: Not a Combination Product 05/13/2011 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph final part352 05/13/2011 Labeler - Temmentec AG (480586411) Registrant - Temmentec AG (480586411) Establishment Name Address ID/FEI Business Operations Temmentec AG 480586411 manufacture(68807-318)