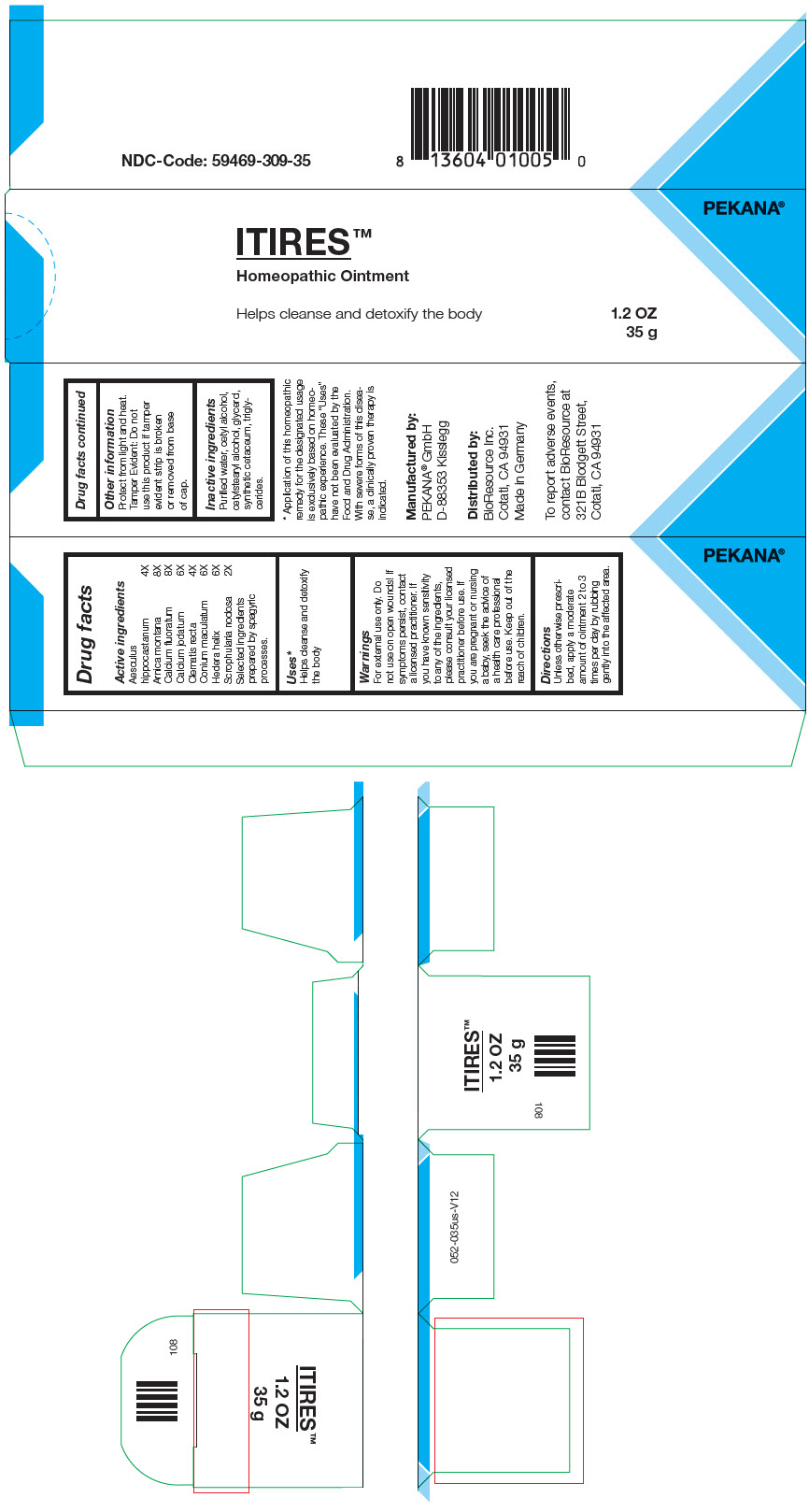
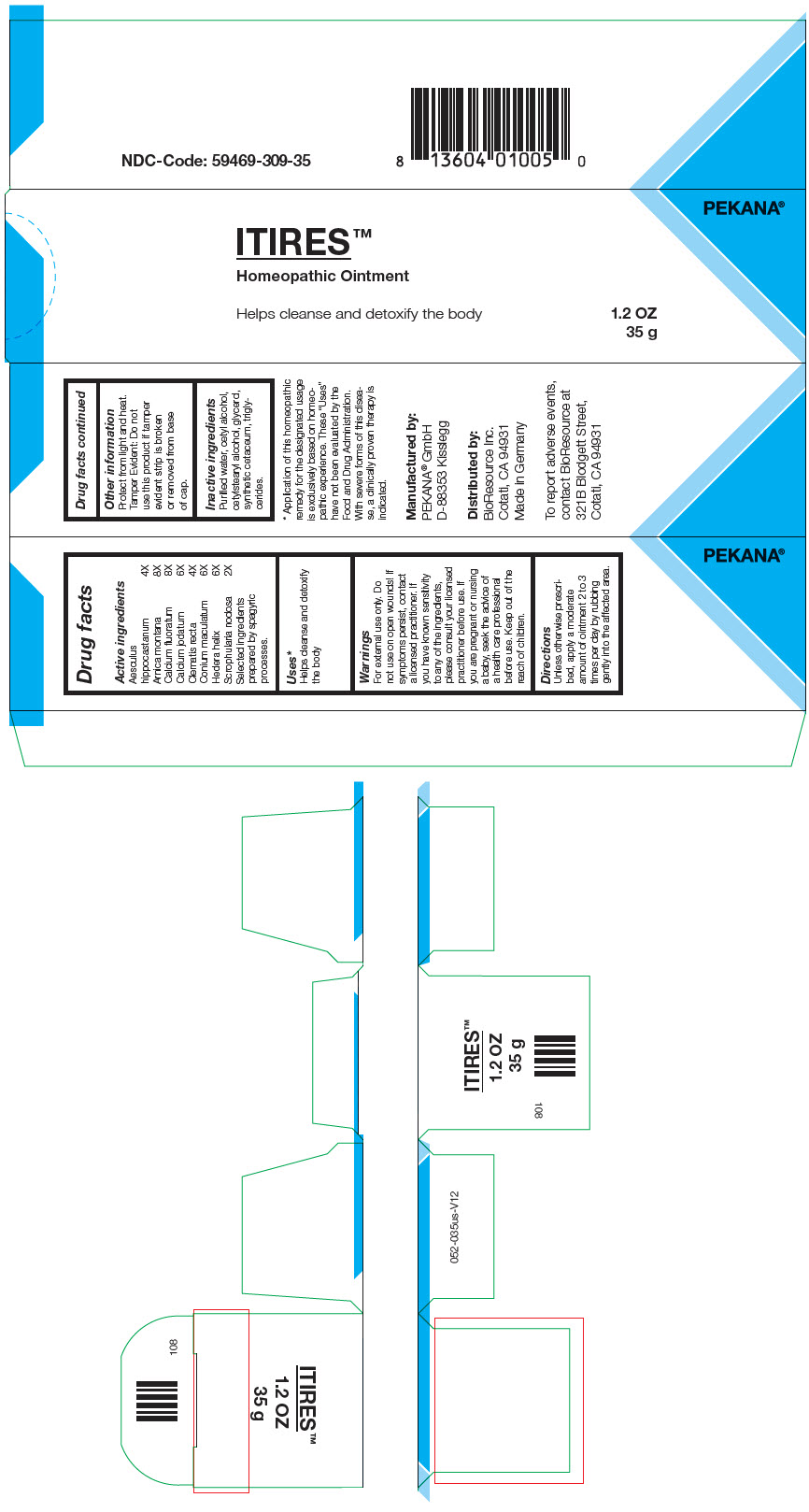
Label: ITIRES- arnica montana root, calcium fluoride, calcium iodide, clematis recta flowering top, scrophularia nodosa, conium maculatum flowering top, horse chestnut, and hedera helix flowering twig ointment
- NDC Code(s): 59469-309-30, 59469-309-35
- Packager: PEKANA Naturheilmittel GmbH
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: unapproved homeopathic
DISCLAIMER: This homeopathic product has not been evaluated by the Food and Drug Administration for safety or efficacy. FDA is not aware of scientific evidence to support homeopathy as effective.
Drug Label Information
Updated December 23, 2022
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- SPL UNCLASSIFIED SECTION
- ACTIVE INGREDIENT
-
Uses1
- 1
- Application of this homeopathic remedy for the designated usage is exclusively based on homeopathic experience. These "Uses" have not been evaluated by the Food and Drug Administration. With severe forms of this disease, a clinically proven therapy is indicated.
-
Warnings
For external use only. Do not use on open wounds! If symptoms persist, contact a licensed practitioner. If you have known sensitivity to any of the ingredients, please consult your licensed practitioner before use. If you are pregnant or nursing a baby, seek the advice of a health care professional before use.
- Directions
- Other information
- Inactive ingredients
- SPL UNCLASSIFIED SECTION
- PRINCIPAL DISPLAY PANEL - 35 g Tube Box
-
INGREDIENTS AND APPEARANCE
ITIRES
arnica montana root, calcium fluoride, calcium iodide, clematis recta flowering top, scrophularia nodosa, conium maculatum flowering top, horse chestnut, and hedera helix flowering twig ointmentProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:59469-309 Route of Administration CUTANEOUS Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Arnica montana Root (UNII: MUE8Y11327) (Arnica montana Root - UNII:MUE8Y11327) Arnica montana Root 8 [hp_X] in 35 g CALCIUM FLUORIDE (UNII: O3B55K4YKI) (FLUORIDE ION - UNII:Q80VPU408O) CALCIUM FLUORIDE 8 [hp_X] in 35 g CALCIUM IODIDE (UNII: 8EKI9QEE2H) (CALCIUM CATION - UNII:2M83C4R6ZB) CALCIUM IODIDE 6 [hp_X] in 35 g Clematis recta Flowering Top (UNII: 396421SP9F) (Clematis recta Flowering Top - UNII:396421SP9F) Clematis recta Flowering Top 4 [hp_X] in 35 g SCROPHULARIA NODOSA WHOLE (UNII: 7H443NUB2T) (SCROPHULARIA NODOSA WHOLE - UNII:7H443NUB2T) SCROPHULARIA NODOSA WHOLE 2 [hp_X] in 35 g Conium maculatum Flowering Top (UNII: Q28R5GF371) (Conium maculatum Flowering Top - UNII:Q28R5GF371) Conium maculatum Flowering Top 6 [hp_X] in 35 g HORSE CHESTNUT (UNII: 3C18L6RJAZ) (HORSE CHESTNUT - UNII:3C18L6RJAZ) HORSE CHESTNUT 4 [hp_X] in 35 g Hedera helix Flowering Twig (UNII: 3D10KUA6BM) (Hedera helix Flowering Twig - UNII:3D10KUA6BM) Hedera helix Flowering Twig 6 [hp_X] in 35 g Inactive Ingredients Ingredient Name Strength MEDIUM-CHAIN TRIGLYCERIDES (UNII: C9H2L21V7U) CETOSTEARYL ALCOHOL (UNII: 2DMT128M1S) Cetyl Alcohol (UNII: 936JST6JCN) SPERMACETI (UNII: P86KHA8V3K) GLYCERIN (UNII: PDC6A3C0OX) WATER (UNII: 059QF0KO0R) Alcohol (UNII: 3K9958V90M) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:59469-309-35 1 in 1 BOX 06/24/2019 1 35 g in 1 TUBE; Type 0: Not a Combination Product 2 NDC:59469-309-30 1 in 1 BOX 06/24/2019 2 30 g in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date Unapproved homeopathic 06/24/2019 Labeler - PEKANA Naturheilmittel GmbH (320344542) Establishment Name Address ID/FEI Business Operations PEKANA Naturheilmittel GmbH 320344542 MANUFACTURE(59469-309)