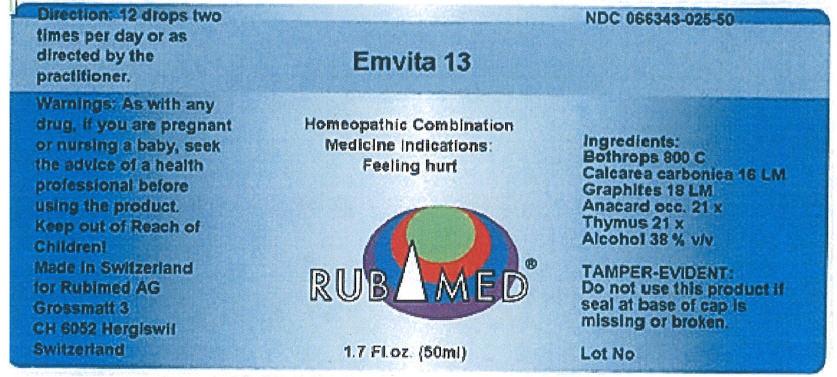
Label: EMVITA 13- bothrops atrox venom, oyster shell calcium carbonate, crude, graphite, anacardium occidentale fruit, and sus scrofa thymus liquid
-
Contains inactivated NDC Code(s)
NDC Code(s): 66343-025-50 - Packager: RUBIMED AG
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: unapproved homeopathic
DISCLAIMER: This homeopathic product has not been evaluated by the Food and Drug Administration for safety or efficacy. FDA is not aware of scientific evidence to support homeopathy as effective.
Drug Label Information
Updated July 9, 2015
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- PURPOSE
- Ingredients
- TAMPER-EVIDENT
- Direction
- Warnings
- PRINCIPAL DISPLAY PANEL - 50 ml Bottle Label
-
INGREDIENTS AND APPEARANCE
EMVITA 13
bothrops atrox venom, oyster shell calcium carbonate, crude, graphite, anacardium occidentale fruit, and sus scrofa thymus liquidProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:66343-025 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Bothrops Atrox Venom (UNII: K0Q6REZ9JC) (Bothrops Atrox Venom - UNII:K0Q6REZ9JC) Bothrops Atrox Venom 800 [hp_C] in 1 mL Oyster shell calcium carbonate, crude (UNII: 2E32821G6I) (Oyster shell calcium carbonate, crude - UNII:2E32821G6I) Oyster shell calcium carbonate, crude 16 [hp_M] in 1 mL Graphite (UNII: 4QQN74LH4O) (Graphite - UNII:4QQN74LH4O) Graphite 18 [hp_M] in 1 mL Anacardium Occidentale Fruit (UNII: 4A10JR4E7E) (Anacardium Occidentale Fruit - UNII:4A10JR4E7E) Anacardium Occidentale Fruit 21 [hp_X] in 1 mL Sus Scrofa Thymus (UNII: 7B69B0BD62) (Sus Scrofa Thymus - UNII:7B69B0BD62) Sus Scrofa Thymus 21 [hp_X] in 1 mL Inactive Ingredients Ingredient Name Strength Alcohol (UNII: 3K9958V90M) 0.38 mL in 1 mL Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:66343-025-50 50 mL in 1 BOTTLE, DROPPER; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date UNAPPROVED HOMEOPATHIC 05/07/2015 Labeler - RUBIMED AG (480582035) Establishment Name Address ID/FEI Business Operations RUBIMED AG 480582035 MANUFACTURE(66343-025) Establishment Name Address ID/FEI Business Operations Omida AG 483268348 MANUFACTURE(66343-025)