Label: SPF 15 - ZINC OXIDE LIP BALM- zinc oxide stick
- NDC Code(s): 63645-182-01
- Packager: OraLabs
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: OTC monograph not final
DISCLAIMER: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
Drug Label Information
Updated July 19, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
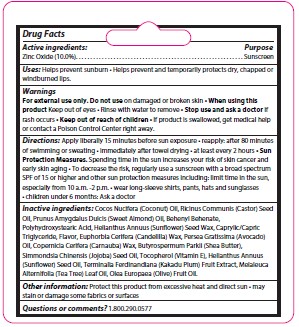
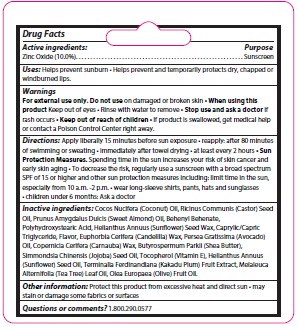
- Active ingredient
- Purpose
- Keep Out of Reach of Children
- Uses
- Warnings
-
Directions
Apply liberally 15 minutes before sun exposure • reapply: after 80 minutes
of swimming or sweating • immediately after towel drying • at least every 2 hours • Sun
Protection Measures. Spending time in the sun increases your risk of skin cancer and
early skin aging • To decrease the risk, regularly use a sunscreen with a broad spectrum
SPF of 15 or higher and other sun protection measures including: limit time in the sun,
especially from 10 a.m. -2 p.m. • wear long-sleeve shirts, pants, hats and sunglasses. Children under 6 months: Ask a doctor.
-
Inactive Ingredients
Cocos Nucifera (Coconut) Oil, Ricinus Communis (Castor) Seed Oil, Prunus Amygdalus Dulcis (Sweet Almond) Oil, Behenyl Behenate, Polyhydroxystearic Acid, Helianthus Annuus (Sunflower) Seed Wax, Caprylic/CapricTriglyceride, Flavor, Euphorbia Cerifera (Candelilla) Wax, Persea Gratissima (Avocado) Oil, Copernicia Cerifera (Carnauba) Wax, Butyrospermum Parkii (Shea Butter),Simmondsia Chinensis (Jojoba) Seed Oil, Tocopherol (Vitamin E), Helianthus Annuus(Sunflower) Seed Oil, Terminalia Ferdinandiana (Kakadu Plum) Fruit Extract, Melaleuca
- Package/Label Principal Display Panel
-
INGREDIENTS AND APPEARANCE
SPF 15 - ZINC OXIDE LIP BALM
zinc oxide stickProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:63645-182 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ZINC OXIDE (UNII: SOI2LOH54Z) (ZINC OXIDE - UNII:SOI2LOH54Z) ZINC OXIDE 100 mg in 1 g Inactive Ingredients Ingredient Name Strength COCONUT OIL (UNII: Q9L0O73W7L) 205 mg in 1 g WHITE WAX (UNII: 7G1J5DA97F) 202 mg in 1 g ALMOND OIL (UNII: 66YXD4DKO9) 170 mg in 1 g CASTOR OIL (UNII: D5340Y2I9G) 170 mg in 1 g Product Characteristics Color WHITE Score Shape Size Flavor Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:63645-182-01 4 g in 1 CONTAINER; Type 0: Not a Combination Product 06/06/2023 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph not final part352 06/06/2023 Labeler - OraLabs (801824756) Registrant - OraLabs (801824756) Establishment Name Address ID/FEI Business Operations OraLabs 801824756 MANUFACTURE(63645-182) , LABEL(63645-182)