Label: BUPIVACAINE HYDROCHLORIDE injection
-
Contains inactivated NDC Code(s)
NDC Code(s): 59923-717-05, 59923-718-05, 59923-719-10, 59923-720-10 - Packager: Areva Pharmaceuticals
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Unapproved drug for use in drug shortage
DISCLAIMER: This drug has not been found by FDA to be safe and effective, and this labeling has not been approved by FDA. For further information about unapproved drugs, click here.
Drug Label Information
Updated January 14, 2021
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
HEALTH CARE PROVIDER LETTER
June 21, 2019
Important Prescribing Information
Subject: Temporary Importation of Bupivacaine Hydrochloride Injection, Ampules to Address Supply Shortage
Dear Health Care Provider:
In order to address ongoing shortages of Bupivacaine Hydrochloride Injection, Areva Pharmaceuticals (Areva), is coordinating with the U.S. Food and Drug Administration (FDA) to make available 5 mL and 10 mL Bupivacaine Hydrochloride Injection 0.25% and 0.5% Single-Dose ampules manufactured by Areva’s supplier, Fisiopharma, in Italy.
At this time, no other entity except Areva is authorized by the FDA to import or distribute Bupivacaine Hydrochloride Injection, 2.5 mg/mL and 5 mg/mL (equivalent to 0.25% and 0.5%), 5 mL and 10 mL Single-Dose Ampules in the United States. The FDA has not approved this product manufactured by Areva’s supplier in Italy.
Effective immediately, and during this temporary period, Areva will offer the following:
Product Name and Description
Size
NDC
Store at
Bupivacaine Hydrochloride Injection,
2.5 mg/mL (equivalent to 0.25%)
5 mL glass ampule
59923-717-05
20°C to 25°C (68°F to 77°F). [See USP Controlled Room Temperature.]
10 mL glass ampule
59923-719-10
Bupivacaine Hydrochloride Injection,
5 mg/mL (equivalent to 0.5%)
5 mL glass ampule
59923-718-05
20°C to 25°C (68°F to 77°F). [See USP Controlled Room Temperature.]
10 mL glass ampule
59923-720-10
There are key differences between the labeling of the FDA approved Bupivacaine Hydrochloride Injection and Areva’s imported Bupivicana Fisiopharma. It is important to note the following:
- Areva’s product is labeled Bupivacaina Fisiopharma 2,5 mg/mL which means 2.5 mg/mL and is equivalent to 0.25% bupivacaine hydrochloride. Bupivacaina Fisiopharma 5 mg/mL is equivalent to 0.5% bupivacaine hydrochloride. We have affixed a sticker with important information on the carton for the imported product. See example below:
CAUTION
Bupivacaine Hydrochloride Injection 0.5%
Each ampulecontains: 25 mg/mL (5 mg/mL)
Preservative-Free
- The barcode on the imported product label may not register with U.S. scanning systems. Institutions should manually input the imported product information into their systems and confirm that the barcode, if scanned, provides correct information. Alternative procedures should be followed to ensure that the correct drug product is being used and administered to individual patients.
- There is a risk of contamination by glass particles when opening the 5 mL and 10 mL Bupivacaine Hydrochloride Injection ampules. To minimize particulate contamination:
- Follow standard aseptic technique and withdraw contents of the ampules with a 5-micron filter needle (American Society of Health-System Pharmacists Guidelines on Compounding Sterile Preparations 2014).
- After withdrawing ampule contents with filter needle, change needle before injection.
Parenteral drug products should be inspected visually for particulate matter and discoloration prior to administration.
A side-by-side comparison of the key differences in the labeling between the FDA approved product and the imported product is displayed in the product comparison table at the end of this letter.
The bupivacaine from Fisiopharma is approved in Italy for intrathecal use and differs from the US-approved Marcaine Spinal in that the Fisiopharma bupivacaine formulation is an isobaric solution and has two presentations: a concentration of 2.5 mg/mL and 5 mg/mL.
Please refer to the FDA-approved package insert for the full prescribing information of Bupivacaine Hydrochloride Injection.
Healthcare providers should report quality problems and all adverse events associated with the use of bupivacaine to Areva at 1-855-853-4760 or fax 1-812-951-1099.
Adverse events or quality problems experienced with the use of this product may also be reported to the FDA’s MedWatch Adverse Event Reporting program either online, by regular mail or by fax.
- Complete and submit the report Online: www.fda.gov/medwatch/report.htm
- Regular mail or Fax: Download form www.fda.gov/MedWatch/getforms.htm or call 1-800-332-1088 to request a reporting form, then complete and return to the address on the pre-addressed form by fax to 1-800-FDA-0178.
To place an order, please contact Areva’s Customer Service by calling 1-812-399-3599.
If you have questions about the information contained in this letter or the use of the imported product, please contact Areva at 1-855-853-4760.
Sincerely,
Victor Swaminathan, R.Ph.
Chief Executive Officer
Areva Pharmaceuticals, Inc.
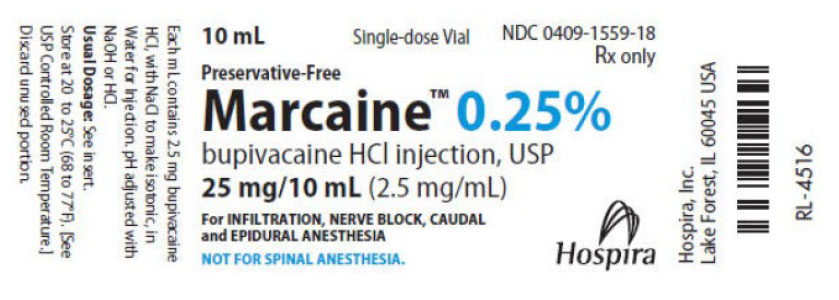
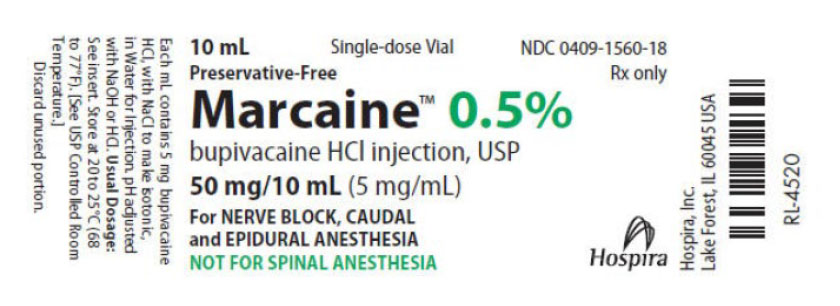
Table 1: Side-by-Side Product Comparison of Bupivacaine Hydrochloride Injection, 0.25% and 0.5%
Imported Product
US FDA Approved Product
Product Name
Bupivacaine Hydrochloride Injection
Marcaine (bupivacaine hydrochloride) Injection, USP
Label
5 mL
Bupivacaina Fisiopharma
2,5 mg/mL injectable solution
Bupivacaine
Batch n.: 00000X
Expiry date: 00/0000
Fisiopharma S.r.l.
10 mL
Bupivacaina Fisiopharma
2,5 mg/mL injectable solution
Bupivacaine
Batch n.: 00000X
Expiry date: 00/0000
Fisiopharma S.r.l.
5 mL
Bupivacaina Fisiopharma
5 mg/mL injectable solution
Bupivacaine
Batch n.: 00000X
Expiry date: 00/0000
Fisiopharma S.r.l.
10 mL
Bupivacaina Fisiopharma
5 mg/mL injectable solution
Bupivacaine
Batch n.: 00000X
Expiry date: 00/0000
Fisiopharma S.r.l.
Composition
Bupivacaine Fisiopharma 2,5 mg/mL solution for injection
Each mL contains:
Active Substance: bupivacaine hydrochloride 2,5 mg
Excipients: sodium chloride, water for injection
Bupivacaine Fisiopharma 5 mg/mL solution for injection
Each mL contains:
Active Substance: bupivacaine hydrochloride 5 mg
Excipients: sodium chloride, water for injection
Preservative-Free
MARCAINE—Sterile isotonic solutions containing sodium chloride. In multiple-dose vials, each mL also contains 1 mg methylparaben as antiseptic preservative. The pH of these solutions is adjusted to between 4 and 6.5 with sodium hydroxide or hydrochloric acid.
Indications
Bupivacaine is indicated in any type of peripheral anesthesia
- Local infiltration to nerve trunk, regional block
- Symphathetic nerve block
- Intravenous retrograde block and intra-arterial block
- Epidural, sacral
- Subarachnoid (spinal) block
Bupivacaine is indicated in any intervention of general surgery, including orthopedics, ophthalmology, otorhinolaryngology, dentistry, obstetrics, and gynecology, dermatology, used either alone or associated with narcosis.
MARCAINE is indicated for the production of local or regional anesthesia or analgesia for surgery, dental and oral surgery procedures, diagnostic and therapeutic procedures, and for obstetrical procedures. Only the 0.25% and 0.5% concentrations are indicated for obstetrical anesthesia.
Contraindications
Hypersensitivity to the active substance, local anesthetic agents of the amide type or to any of the excipients.
The use of bupivacaine should be avoided in patients with ascertained or suspected pregnancy. Cases of cardiac arrest have been reported following the use of bupivacaine in epidural anesthesia in laboring women; in most cases, this occurred following the use of the 0.75% solution which, as a consequence, should be avoided in epidural anesthesia in obstetrics. This concentration should be used for the surgical procedures requiring a high degree and prolonged effect of muscle relaxation.
The product is also contraindicated in paracervical block and intravenous regional anesthesia (Bier’s Block).
MARCAINE is contraindicated in obstetrical paracervical block anesthesia. Its use in this technique has resulted in fetal bradycardia and death.
MARCAINE is contraindicated in patients with a known hypersensitivity to it or to any local anesthetic agent of the amide-type or to other components of MARCAINE solutions.
Precautions
Immediate availability of the drugs equipping and of the proper emergency staff must be ensured as the cases of serious reactions and sometimes with fatal results, although rarely, have been reported following the use of local anesthetics even in the absence of hypersensitivity.
The total dose to use must be determined by evaluating the age, physical status and the main anamnestic data of the patient.
The specific weight of bupivacaine 2.5 mg / ml or 5 mg / ml is 1.006 at 20 ° C and 0.997 at 37 ° C.
The product must be used with absolute caution in subjects undergoing the treatment with drugs that inhibit MAO or with tricyclic antidepressants. Before use, the doctor should check the circulatory system conditions of the patient to treat.
Any overdose of anesthetic should be avoided, and the time interval between two maximum doses should be at least 24 hours.
However, the lowest dosage and concentration needed to provide effective anaesthesia should be administered. To avoid undesirable effects following an accidental intravenous or intrathecal injection, it is recommended to perform the administration of a preceding test dose of Bupivacaine containing adrenaline.
The administration is carried out in small doses approximately 10 seconds after a preventive aspiration has been done.
If infiltrations are practiced for local anesthesia in areas lacking the possibility of collateral circulation (fingers, penile root, etc.), it is a precautionary measure to use anesthetic without vasoconstrictor to avoid ischemic necrosis.
Especially when highly vascularity areas have to be infiltrated, it is recommended to wait 2 minutes prior to proceed effectively with the loco-regional blocking.
During the administration, the patient must be closely observed and administration promptly suspended at the first sign of alarm (such as the sensory changes).
General: The safety and effectiveness of local anesthetics depend on proper dosage, correct technique, adequate precautions, and readiness for emergencies. Resuscitative equipment, oxygen, and other resuscitative drugs should be available for immediate use (See WARNINGS, ADVERSE REACTIONS, and OVERDOSAGE). During major regional nerve blocks, the patient should have intravenous fluids running via an indwelling catheter to assure a functioning intravenous pathway. The lowest dosage of local anesthetic that results in effective anesthesia should be used to avoid high plasma levels and serious adverse effects. The rapid injection of a large volume of local anesthetic solution should be avoided and fractional (incremental) doses should be used when feasible.
Epidural Anesthesia: During epidural administration of MARCAINE, 0.5% and 0.75% solutions should be administered in incremental doses of 3 mL to 5 mL with sufficient time between doses to detect toxic manifestations of unintentional intravascular or intrathecal injection. Injections should be made slowly, with frequent aspirations before and during the injection to avoid intravascular injection. Syringe aspirations should also be performed before and during each supplemental injection in continuous (intermittent) catheter techniques. An intravascular injection is still possible even if aspirations for blood are negative.
During the administration of epidural anesthesia, it is recommended that a test dose be administered initially and the effects monitored before the full dose is given. When using a “continuous” catheter technique, test doses should be given prior to both the original and all reinforcing doses, because plastic tubing in the epidural space can migrate into a blood vessel or through the dura. When clinical conditions permit, the test dose should contain epinephrine (10 mcg to 15 mcg has been suggested) to serve as a warning of unintended intravascular injection. If injected into a blood vessel, this amount of epinephrine is likely to produce a transient “epinephrine response” within 45 seconds, consisting of an increase in heart rate and/or systolic blood pressure, circumoral pallor, palpitations, and nervousness in the unsedated patient. The sedated patient may exhibit only a pulse rate increase of 20 or more beats per minute for 15 or more seconds. Therefore, following the test dose, the heart rate should be monitored for a heart rate increase. Patients on beta-blockers may not manifest changes in heart rate, but blood pressure monitoring can detect a transient rise in systolic blood pressure. The test dose should also contain 10 mg to 15 mg of MARCAINE or an equivalent amount of another local anesthetic to detect an unintended intrathecal administration. This will be evidenced within a few minutes by signs of spinal block (e.g., decreased sensation of the buttocks, paresis of the legs, or, in the sedated patient, absent knee jerk). The Test Dose formulation of MARCAINE contains 15 mg of bupivacaine and 15 mcg of epinephrine in a volume of
3 mL. An intravascular or subarachnoid injection is still possible even if results of the test dose are negative. The test dose itself may produce a systemic toxic reaction, high spinal or epinephrine-induced cardiovascular effects.
Injection of repeated doses of local anesthetics may cause significant increases in plasma levels with each repeated dose due to slow accumulation of the drug or its metabolites, or to slow metabolic degradation. Tolerance to elevated blood levels varies with the status of the patient. Debilitated, elderly patients and acutely ill patients should be given reduced doses commensurate with their age and physical status. Local anesthetics should also be used with caution in patients with hypotension or heartblock.
Careful and constant monitoring of cardiovascular and respiratory (adequacy of ventilation) vital signs and the patient’s state of consciousness should be performed after each local anesthetic injection. It should be kept in mind at such times that restlessness, anxiety, incoherent speech, lightheadedness, numbness and tingling of the mouth and lips, metallic taste, tinnitus, dizziness, blurred vision, tremors, twitching, depression, or drowsiness may be early warning signs of CNS toxicity.
Local anesthetic solutions containing a vasoconstrictor should be used cautiously and in carefully restricted quantities in areas of the body supplied by end arteries or having otherwise compromised blood supply such as digits, nose, external ear, or penis. Patients with hypertensive vascular disease may exhibit exaggerated vasoconstrictor response. Ischemic injury or necrosis may result.
Because amide-local anesthetics such as MARCAINE are metabolized by the liver, these drugs, especially repeat doses, should be used cautiously in patients with hepatic disease. Patients with severe hepatic disease, because of their inability to metabolize local anesthetics normally, are at a greater risk of developing toxic plasma concentrations. Local anesthetics should also be used with caution in patients with impaired cardiovascular function because they may be less able to compensate for functional changes associated with the prolongation of AV conduction produced by these drugs.
Serious dose-related cardiac arrhythmias may occur if preparations containing a vasoconstrictor such as epinephrine are employed in patients during or following the administration of potent inhalation anesthetics. In deciding whether to use these products concurrently in the same patient, the combined action of both agents upon the myocardium, the concentration and volume of vasoconstrictor used, and the time since injection, when applicable, should be taken into account.
Many drugs used during the conduct of anesthesia are considered potential triggering agents for familial malignant hyperthermia. Because it is not known whether amide-type local anesthetics may trigger this reaction and because the need for supplemental general anesthesia cannot be predicted in advance, it is suggested that a standard protocol for management should be available. Early unexplained signs of tachycardia, tachypnea, labile blood pressure, and metabolic acidosis may precede temperature elevation. Successful outcome is dependent on early diagnosis, prompt discontinuance of the suspect triggering agent(s) and prompt institution of treatment, including oxygen therapy, indicated supportive measures and dantrolene (Consult dantrolene sodium intravenous package insert before using).
Use in Head and Neck Area: Small doses of local anesthetics injected into the head and neck area, including retrobulbar, dental, and stellate ganglion blocks, may produce adverse reactions similar to systemic toxicity seen with unintentional intravascular injections of larger doses. The injection procedures require the utmost care. Confusion, convulsions, respiratory depression, and/or respiratory arrest, and cardiovascular stimulation or depression have been reported. These reactions may be due to intra-arterial injection of the local anesthetic with retrograde flow to the cerebral circulation. They may also be due to puncture of the dural sheath of the optic nerve during retrobulbar block with diffusion of any local anesthetic along the subdural space to the midbrain. Patients receiving these blocks should have their circulation and respiration monitored and be constantly observed. Resuscitative equipment and personnel for treating adverse reactions should be immediately available. Dosage recommendations should not be exceeded (See DOSAGE AND ADMINISTRATION).
Use in Ophthalmic Surgery: Clinicians who perform retrobulbar blocks should be aware that there have been reports of respiratory arrest following local anesthetic injection. Prior to retrobulbar block, as with all other regional procedures, the immediate availability of equipment, drugs, and personnel to manage respiratory arrest or depression, convulsions, and cardiac stimulation or depression should be assured (see also WARNINGS and Use In Head and Neck Area, above). As with other anesthetic procedures, patients should be constantly monitored following ophthalmic blocks for signs of these adverse reactions, which may occur following relatively low total doses.
A concentration of 0.75% bupivacaine is indicated for retrobulbar block; however, this concentration is not indicated for any other peripheral nerve block, including the facial nerve, and not indicated for local infiltration, including the conjunctiva (see INDICATIONS AND USAGE and PRECAUTIONS, General). Mixing MARCAINE with other local anesthetics is not recommended because of insufficient data on the clinical use of such mixtures.
When MARCAINE 0.75% is used for retrobulbar block, complete corneal anesthesia usually precedes onset of clinically acceptable external ocular muscle akinesia. Therefore, presence of akinesia rather than anesthesia alone should determine readiness of the patient for surgery.
Use in Dentistry: Because of the long duration of anesthesia, when MARCAINE 0.5% with epinephrine is used for dental injections, patients should be cautioned about the possibility of inadvertent trauma to tongue, lips, and buccal mucosa and advised not to chew solid foods or test the anesthetized area by biting or probing.
Information for Patients: When appropriate, patients should be informed in advance that they may experience temporary loss of sensation and motor activity, usually in the lower half of the body, following proper administration of caudal or epidural anesthesia. Also, when appropriate, the physician should discuss other information including adverse reactions in the package insert of MARCAINE.
Patients receiving dental injections of MARCAINE should be cautioned not to chew solid foods or test the anesthetized area by biting or probing until anesthesia has worn off (up to 7 hours).
Inform patients that use of local anesthetics may cause methemoglobinemia, a serious condition that must be treated promptly. Advise patients or caregivers to seek immediate medical attention if they or someone in their care experience the following signs or symptoms: pale, gray, or blue colored skin (cyanosis); headache; rapid heart rate; shortness of breath; lightheadedness; or fatigue.
Carcinogenesis, Mutagenesis, Impairment of Fertility: Long-term studies in animals to evaluate the carcinogenic potential of bupivacaine hydrochloride have not been conducted. The mutagenic potential and the effect on fertility of bupivacaine hydrochloride have not been determined.
Pregnancy: There are no adequate and well-controlled studies in pregnant women. MARCAINE should be used during pregnancy only if the potential benefit justifies the potential risk to the fetus. Bupivacaine hydrochloride produced developmental toxicity when administered subcutaneously to pregnant rats and rabbits at clinically relevant doses. This does not exclude the use of MARCAINE at term for obstetrical anesthesia or analgesia (See Labor and Delivery).
Bupivacaine hydrochloride was administered subcutaneously to rats at doses of 4.4, 13.3, & 40 mg/kg and to rabbits at doses of 1.3, 5.8, & 22.2 mg/kg during the period of organogenesis (implantation to closure of the hard palate). The high doses are comparable to the daily maximum recommended human dose (MRHD) of 400 mg/day on a mg/m2 body surface area (BSA) basis. No embryo-fetal effects were observed in rats at the high dose which caused increased maternal lethality. An increase in embryo-fetal deaths was observed in rabbits at the high dose in the absence of maternal toxicity with the fetal No Observed Adverse Effect Level representing approximately 1/5th the MRHD on a BSA basis.
In a rat pre- and post-natal development study (dosing from implantation through weaning) conducted at subcutaneous doses of 4.4, 13.3, & 40 mg/kg, decreased pup survival was observed at the high dose. The high dose is comparable to the daily MRHD of 400 mg/day on a BSA basis.
Labor and Delivery: SEE BOXED WARNING REGARDING OBSTETRICAL USE OF 0.75% MARCAINE.
MARCAINE is contraindicated for obstetrical paracervical block anesthesia.
Local anesthetics rapidly cross the placenta, and when used for epidural, caudal, or pudendal block anesthesia, can cause varying degrees of maternal, fetal, and neonatal toxicity (See CLINICAL PHARMACOLOGY , Pharmacokinetics ). The incidence and degree of toxicity depend upon the procedure performed, the type, and amount of drug used, and the technique of drug administration. Adverse reactions in the parturient, fetus, and neonate involve alterations of the CNS, peripheral vascular tone, and cardiac function.
Maternal hypotension has resulted from regional anesthesia. Local anesthetics produce vasodilation by blocking sympathetic nerves. Elevating the patient’s legs and positioning her on her left side will help prevent decreases in blood pressure. The fetal heart rate also should be monitored continuously and electronic fetal monitoring is highly advisable.
Epidural, caudal, or pudendal anesthesia may alter the forces of parturition through changes in uterine contractility or maternal expulsive efforts. Epidural anesthesia has been reported to prolong the second stage of labor by removing the parturient’s reflex urge to bear down or by interfering with motor function. The use of obstetrical anesthesia may increase the need for forceps assistance.
The use of some local anesthetic drug products during labor and delivery may be followed by diminished muscle strength and tone for the first day or two of life. This has not been reported with bupivacaine.
It is extremely important to avoid aortocaval compression by the gravid uterus during administration of regional block to parturients. To do this, the patient must be maintained in the left lateral decubitus position or a blanket roll or sandbag may be placed beneath the right hip and gravid uterus displaced to the left.
Nursing Mothers: Bupivacaine has been reported to be excreted in human milk suggesting that the nursing infant could be theoretically exposed to a dose of the drug. Because of the potential for serious adverse reactions in nursing infants from bupivacaine, a decision should be made whether to discontinue nursing or not administer bupivacaine, taking into account the importance of the drug to the mother.
Pediatric Use: Until further experience is gained in pediatric patients younger than 12 years, administration of MARCAINE in this age group is not recommended. Continuous infusions of bupivacaine in children have been reported to result in high systemic levels of bupivacaine and seizures; high plasma levels may also be associated with cardiovascular abnormalities (See WARNINGS, PRECAUTIONS, and OVERDOSAGE).
Geriatric Use: Patients over 65 years, particularly those with hypertension, may be at increased risk for developing hypotension while undergoing anesthesia with MARCAINE (See ADVERSE REACTIONS).
Elderly patients may require lower doses of MARCAINE (See PRECAUTIONS, Epidural Anesthesia and DOSAGE AND ADMINISTRATION ).
In clinical studies, differences in various pharmacokinetic parameters have been observed between elderly and younger patients (See CLINICAL PHARMACOLOGY).
This product is known to be substantially excreted by the kidney, and the risk of toxic reactions to this drug may be greater in patients with impaired renal function. Because elderly patients are more likely to have decreased renal function, care should be taken in dose selection, and it may be useful to monitor renal function (See CLINICAL PHARMACOLOGY).
Interactions
Interactions with other drugs are not known. As already mentioned, particular care must be taken when using the drug in subjects undergoing the treatment with the MAO inhibitors or with tricyclic antidepressants.
Clinically Significant Drug Interactions: The administration of local anesthetic solutions containing epinephrine or norepinephrine to patients receiving monoamine oxidase inhibitors or tricyclic antidepressants may produce severe, prolonged hypertension. Concurrent use of these agents should generally be avoided. In situations when concurrent therapy is necessary, careful patient monitoring is essential.
Concurrent administration of vasopressor drugs and of ergot-type oxytocic drugs may cause severe, persistent hypertension or cerebrovascular accidents.
Phenothiazines and butyrophenones may reduce or reverse the pressor effect of epinephrine.
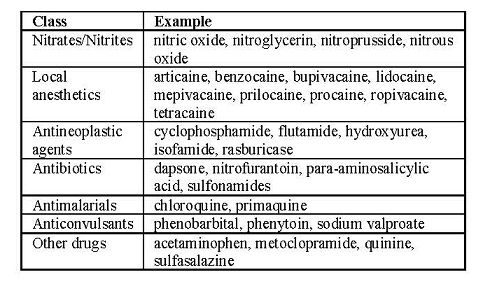
Patients who are administered local anesthetics are at increased risk of developing methemoglobinemia when concurrently exposed to following drugs, which could include other local anesthetics:
Examples of Drugs Associated with Methemoglobinemia:
Warnings
Pregnancy and breastfeeding
Do not use the drug in patients with ascertained or suspected pregnancy.
LOCAL ANESTHETICS SHOULD ONLY BE EMPLOYED BY CLINICIANS WHO ARE WELL VERSED IN DIAGNOSIS AND MANAGEMENT OF DOSE-RELATED TOXICITY AND OTHER ACUTE EMERGENCIES WHICH MIGHT ARISE FROM THE BLOCK TO BE EMPLOYED, AND THEN ONLY AFTER INSURING THE IMMEDIATE AVAILABILITY OF OXYGEN, OTHER RESUSCITATIVE DRUGS, CARDIOPULMONARY RESUSCITATIVE EQUIPMENT, AND THE PERSONNEL RESOURCES NEEDED FOR PROPER MANAGEMENT OF TOXIC REACTIONS AND RELATED EMERGENCIES. (See also ADVERSE REACTIONS, PRECAUTIONS, and OVERDOSAGE.) DELAY IN PROPER MANAGEMENT OF DOSE-RELATED TOXICITY, UNDERVENTILATION FROM ANY CAUSE, AND/OR ALTERED SENSITIVITY MAY LEAD TO THE DEVELOPMENT OF ACIDOSIS, CARDIAC ARREST AND, POSSIBLY, DEATH.
Methemoglobinemia: Cases of methemoglobinemia have been reported in association with local anesthetic use. Although all patients are at risk for methemoglobinemia, patients with glucose-6-phosphate dehydrogenase deficiency, congenital or idiopathic methemoglobinemia, cardiac or pulmonary compromise, infants under 6 months of age, and concurrent exposure to oxidizing agents or their metabolites are more susceptible to developing clinical manifestations of the condition. If local anesthetics must be used in these patients, close monitoring for symptoms and signs of methemoglobinemia is recommended.
Signs of methemoglobinemia may occur immediately or may be delayed some hours after exposure, and are characterized by a cyanotic skin discoloration and/or abnormal coloration of the blood.
Methemoglobin levels may continue to rise; therefore, immediate treatment is required to avert more serious CNS and cardiovascular adverse effects, including seizures, coma, arrhythmias, and death.
Discontinue MARCAINE and any other oxidizing agents. Depending on the severity of the signs and symptoms, patients may respond to supportive care, i.e., oxygen therapy, hydration. A more severe clinical presentation may require treatment with methylene blue, exchange transfusion, or hyperbaric oxygen.
Local anesthetic solutions containing antimicrobial preservatives, i.e., those supplied in multiple-dose vials, should not be used for epidural or caudal anesthesia because safety has not been established with regard to intrathecal injection, either intentionally or unintentionally, of such preservatives.
Intra-articular infusions of local anesthetics following arthroscopic and other surgical procedures is an unapproved use, and there have been post-marketing reports of chondrolysis in patients receiving such infusions. The majority of reported cases of chondrolysis have involved the shoulder joint; cases of gleno- humeral chondrolysis have been described in pediatric and adult patients following intra-articular infusions of local anesthetics with and without epinephrine for periods of 48 to 72 hours. There is insufficient information to determine whether shorter infusion periods are not associated with these findings. The time of onset of symptoms, such as joint pain, stiffness and loss of motion can be variable, but may begin as early as the 2nd month after surgery. Currently, there is no effective treatment for chondrolysis; patients who experienced chondrolysis have required additional diagnostic and therapeutic procedures and some required arthroplasty or shoulder replacement.
It is essential that aspiration for blood or cerebrospinal fluid (where applicable) be done prior to injecting any local anesthetic, both the original dose and all subsequent doses, to avoid intravascular or subarachnoid injection. However, a negative aspiration does not ensure against an intravascular or subarachnoid injection.
MARCAINE with epinephrine 1:200,000 or other vasopressors should not be used concomitantly with ergot-type oxytocic drugs, because a severe persistent hypertension may occur. Likewise, solutions of MARCAINE containing a vasoconstrictor, such as epinephrine, should be used with extreme caution in patients receiving monoamineoxidase inhibitors (MAOI) or antidepressants of the triptyline or imipramine types, because severe prolonged hypertension may result.
Until further experience is gained in pediatric patients younger than 12 years, administration of MARCAINE in this age group is not recommended.
Mixing or the prior or intercurrent use of any other local anesthetic with MARCAINE cannot be recommended because of insufficient data on the clinical use of such mixtures.
There have been reports of cardiac arrest and death during the use of MARCAINE for intravenous regional anesthesia (Bier Block). Information on safe dosages and techniques of administration of MARCAINE in this procedure is lacking. Therefore, MARCAINE is not recommended for use in this technique.
MARCAINE with epinephrine 1:200,000 contains sodium metabisulfite, a sulfite that may cause allergic- type reactions including anaphylactic symptoms and life-threatening or less severe asthmatic episodes in certain susceptible people. The overall prevalence of sulfite sensitivity in the general population is unknown and probably low. Sulfite sensitivity is seen more frequently in asthmatic than in nonasthmatic people. Single-dose ampuls and single-dose vials of MARCAINE without epinephrine do not contain sodium metabisulfite.
Dosage and Administration
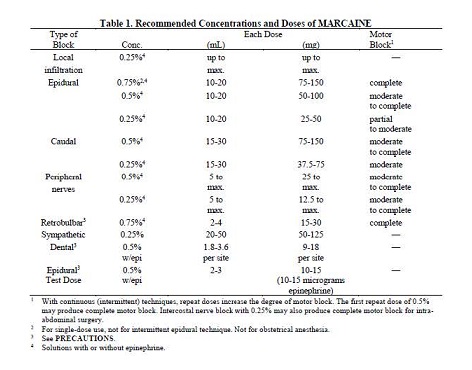
Bupivacaine is usually given in the lowest dosage that varies depending upon the area to anaesthetized, from 2-3 mg to 100-150 mg. The dosages in the following table are recommended as a guide:
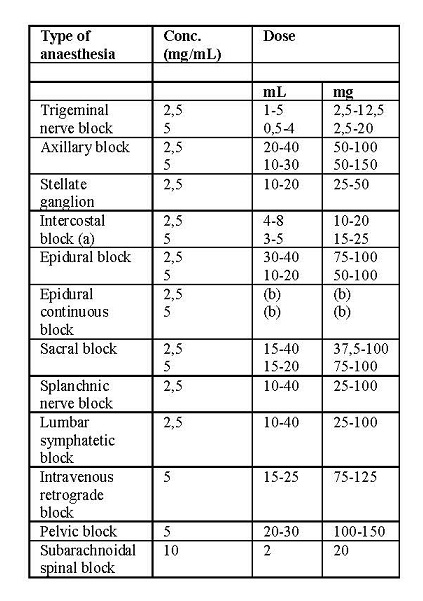
(a): concentration per each intercostal space
(b): start with 10 mL and continue with 3-5-8 ml every 4 – 6 hours, depending upon the area to be anaesthetized and the age of the patient.
Attention: The ampoules do not contain preservatives and should be used for a single administration. Any unused solution should be discarded.
The maximum recommended dosage for an adult at a single occasion should not exceed 150 mg, corresponding to 30 ml of the 5 mg / ml solution and to 60 ml of the 2.5 mg / ml solution; generally, the maximum recommended dose, for both adults and children, is 2 mg / kg per single administration.
In protracted analgesic therapy, doses ranging from 0.25 to 1 mg / kg of body weight are usually given and may be repeated 2-3 times in 24 hours.
The dose of any local anesthetic administered varies with the anesthetic procedure, the area to be anesthetized, the vascularity of the tissues, the number of neuronal segments to be blocked, the depth of anesthesia and degree of muscle relaxation required, the duration of anesthesia desired, individual tolerance, and the physical condition of the patient. The smallest dose and concentration required to produce the desired result should be administered. Dosages of MARCAINE should be reduced for elderly and/or debilitated patients and patients with cardiac and/or liver disease. The rapid injection of a large volume of local anesthetic solution should be avoided and fractional (incremental) doses should be used when feasible.
For specific techniques and procedures, refer to standard textbooks.
There have been adverse event reports of chondrolysis in patients receiving intra-articular infusions of local anesthetics following arthroscopic and other surgical procedures. MARCAINE is not approved for this use (see WARNINGS and DOSAGE AND ADMINISTRATION).
In recommended doses, MARCAINE produces complete sensory block, but the effect on motor function differs among the three concentrations.
0.25%―when used for caudal, epidural, or peripheral nerve block, produces incomplete motor block. Should be used for operations in which muscle relaxation is not important, or when another means of providing muscle relaxation is used concurrently. Onset of action may be slower than with the 0.5% or 0.75% solutions.
0.5%―provides motor blockade for caudal, epidural, or nerve block, but muscle relaxation may be inadequate for operations in which complete muscle relaxation is essential.
The duration of anesthesia with MARCAINE is such that for most indications, a single-dose is sufficient.
Maximum dosage limit must be individualized in each case after evaluating the size and physical status of the patient, as well as the usual rate of systemic absorption from a particular injection site. Most experience to date is with single-doses of MARCAINE up to 225 mg with epinephrine 1:200,000 and 175 mg without epinephrine; more or less drug may be used depending on individualization of each case.
These doses may be repeated up to once every three hours. In clinical studies to date, total daily doses have been up to 400 mg. Until further experience is gained, this dose should not be exceeded in 24 hours. The duration of anesthetic effect may be prolonged by the addition of epinephrine.
The dosages in Table 1 have generally proved satisfactory and are recommended as a guide for use in the average adult. These dosages should be reduced for elderly or debilitated patients. Until further experience is gained, MARCAINE is not recommended for pediatric patients younger than 12 years.
MARCAINE is contraindicated for obstetrical paracervical blocks, and is not recommended for intravenous regional anesthesia (Bier Block).
Use in Epidural Anesthesia: During epidural administration of MARCAINE, 0.5% and 0.75% solutions should be administered in incremental doses of 3 mL to 5 mL with sufficient time between doses to detect toxic manifestations of unintentional intravascular or intrathecal injection. In obstetrics, only the 0.5% and 0.25% concentrations should be used; incremental doses of 3 mL to 5 mL of the 0.5% solution not exceeding 50 mg to 100 mg at any dosing interval are recommended. Repeat doses should be preceded by a test dose containing epinephrine if not contraindicated. Use only the single-dose ampuls and single-dose vials for caudal or epidural anesthesia; the multiple-dose vials contain a preservative and therefore should not be used for these procedures.
Test Dose for Caudal and Lumbar Epidural Blocks: The Test Dose of MARCAINE (0.5% bupivacaine with 1:200,000 epinephrine in a 3 mL ampul) is recommended for use as a test dose when clinical conditions permit prior to caudal and lumbar epidural blocks. This may serve as a warning of unintended intravascular or subarachnoid injection (See PRECAUTIONS). The pulse rate and other signs should be monitored carefully immediately following each test dose administration to detect possible intravascular injection, and adequate time for onset of spinal block should be allotted to detect possible intrathecal injection. An intravascular or subarachnoid injection is still possible even if results of the test dose are negative. The test dose itself may produce a systemic toxic reaction, high spinal or cardiovascular effects from the epinephrine (See WARNINGS and OVERDOSAGE).
Use in Dentistry: The 0.5% concentration with epinephrine is recommended for infiltration and block injection in the maxillary and mandibular area when a longer duration of local anesthetic action is desired, such as for oral surgical procedures generally associated with significant postoperative pain. The average dose of 1.8 mL (9 mg) per injection site will usually suffice; an occasional second dose of 1.8 mL (9 mg) may be used if necessary to produce adequate anesthesia after making allowance for 2 to 10 minutes onset time (See CLINICAL PHARMACOLOGY). The lowest effective dose should be employed and time should be allowed between injections; it is recommended that the total dose for all injection sites, spread out over a single dental sitting, should not ordinarily exceed 90 mg for a healthy adult patient (ten 1.8 mL injections of 0.5% MARCAINE with epinephrine). Injections should be made slowly and with frequent aspirations. Until further experience is gained, MARCAINE in dentistry is not recommended for pediatric patients younger than 12 years.
Unused portions of solution not containing preservatives, i.e., those supplied in single-dose ampuls and single-dose vials, should be discarded following initial use.
This product should be inspected visually for particulate matter and discoloration prior to administration whenever solution and container permit. Solutions which are discolored or which contain particulate matter should not be administered.
Overdosage
At the onset of the first signs of overdose, the administration of the product should immediately be discontinued and the patient placed in a horizontal position to ensure a possible loss of functional airways. If breathing difficulties occur, the assisted ventilation is required (the Ambu balloon can also be used in emergency). The use of bulbar analeptics is not recommended as they increase oxygen consumption. The appearance of convulsive manifestations can be controlled with diazepam given in a vein (10-20 mg); barbiturates that can accentuate bulbar depression are not recommended.
To support the circulatory system, intravenous cortisone can be used; diluted solutions of α-β-stimulants with vasoconstrictor action (mephentermine, metaraminol and others) or atropine sulfate can be added. If acidosis should occur, appropriate solutions of sodium bicarbonate may be given intravenously.
Acute emergencies from local anesthetics are generally related to high plasma levels encountered during therapeutic use of local anesthetics or to unintended subarachnoid injection of local anesthetic solution (See ADVERSE REACTIONS, WARNINGS, and PRECAUTIONS).
Management of Local Anesthetic Emergencies: The first consideration is prevention, best accomplished by careful and constant monitoring of cardiovascular and respiratory vital signs and the patient’s state of consciousness after each local anesthetic injection. At the first sign of change, oxygen should be administered.
The first step in the management of systemic toxic reactions, as well as underventilation or apnea due to unintentional subarachnoid injection of drug solution, consists of immediate attention to the establishment and maintenance of a patent airway and effective assisted or controlled ventilation with 100% oxygen with a delivery system capable of permitting immediate positive airway pressure by mask. This may prevent convulsions if they have not already occurred.
If necessary, use drugs to control the convulsions. A 50 mg to 100 mg bolus intravenous injection of succinylcholine will paralyze the patient without depressing the CNS or cardiovascular system and facilitate ventilation. A bolus intravenous dose of 5 mg to 10 mg of diazepam or 50 mg to 100 mg of thiopental will permit ventilation and counteract CNS stimulation, but these drugs also depress CNS, respiratory, and cardiac function, add to postictal depression and may result in apnea. Intravenous barbiturates, anticonvulsant agents, or muscle relaxants should only be administered by those familiar with their use. Immediately after the institution of these ventilatory measures, the adequacy of the circulation should be evaluated. Supportive treatment of circulatory depression may require administration of intravenous fluids, and when appropriate, a vasopressor dictated by the clinical situation (such as ephedrine or epinephrine to enhance myocardial contractile force).
Endotracheal intubation, employing drugs and techniques familiar to the clinician, may be indicated after initial administration of oxygen by mask if difficulty is encountered in the maintenance of a patent airway, or if prolonged ventilatory support (assisted or controlled) is indicated.
Recent clinical data from patients experiencing local anesthetic-induced convulsions demonstrated rapid development of hypoxia, hypercarbia, and acidosis with bupivacaine within a minute of the onset of convulsions. These observations suggest that oxygen consumption and carbon dioxide production are greatly increased during local anesthetic convulsions and emphasize the importance of immediate and effective ventilation with oxygen which may avoid cardiac arrest.
If not treated immediately, convulsions with simultaneous hypoxia, hypercarbia, and acidosis plus myocardial depression from the direct effects of the local anesthetic may result in cardiac arrhythmias, bradycardia, asystole, ventricular fibrillation, or cardiac arrest. Respiratory abnormalities, including apnea, may occur. Underventilation or apnea due to unintentional subarachnoid injection of local anesthetic solution may produce these same signs and also lead to cardiac arrest if ventilatory support is not instituted. If cardiac arrest should occur, successful outcome may require prolonged resuscitative efforts.
The supine position is dangerous in pregnant women at term because of aortocaval compression by the gravid uterus. Therefore during treatment of systemic toxicity, maternal hypotension or fetal bradycardia following regional block, the parturient should be maintained in the left lateral decubitus position if possible, or manual displacement of the uterus off the great vessels be accomplished.
The mean seizure dosage of bupivacaine in rhesus monkeys was found to be 4.4 mg/kg with mean arterial plasma concentration of 4.5 mcg/mL. The intravenous and subcutaneous LD50 in mice is 6 mg/kg to
8 mg/kg and 38 mg/kg to 54 mg/kg respectively.
Adverse Reactions
Toxic and allergic reactions due to both anaesthetic and vasoconstrictor can occur. Due to an excessive increase in bupivacaine blood concentration caused by incorrect dosage or improper techniques, phenomena of central nerve stimulation are most commonly reported with excitement, tremors, disorientation, vertigo, mydriasis, increased metabolism and body temperature, and due to very high doses, trismus and convulsions.
If the brain stem is affected due to the stimulation of the cardiovascular, respiratory and emetic centers, sweating phenomena, arrhythmias, hypertension, tachypnea, nausea, vomiting and bronchodilation may occur
The cardiovascular system can be affected by a conductional capacity reduction and depression of cardiac contraction. A secondary hypotensive state due to vasodilatation can also be observed.
Allergic reactions mostly occur in hypersensitive subjects, however many cases with no individual hypersensitivity to the anamnesis are reported. Local manifestations include different types of skin rashes, urticaria, itching; general manifestations include bronchospasm, laryngeal edema, cardiovascular collapse, up to an anaphylactic reaction.
Compliance with the instructions contained in the leaflet reduces the risk of undesirable effects.
It is important to consult the doctor or pharmacist about the appearance of any undesirable effect even if not mentioned in the package leaflet.
Reactions to MARCAINE are characteristic of those associated with other amide-type local anesthetics. A major cause of adverse reactions to this group of drugs is excessive plasma levels, which may be due to overdosage, unintentional intravascular injection, or slow metabolic degradation.
The most commonly encountered acute adverse experiences which demand immediate counter-measures are related to the CNS and the cardiovascular system. These adverse experiences are generally dose related and due to high plasma levels which may result from overdosage, rapid absorption from the injection site, diminished tolerance, or from unintentional intravascular injection of the local anesthetic solution. In addition to systemic dose-related toxicity, unintentional subarachnoid injection of drug during the intended performance of caudal or lumbar epidural block or nerve blocks near the vertebral column (especially in the head and neck region) may result in underventilation or apnea (“Total or High Spinal”). Also, hypotension due to loss of sympathetic tone and respiratory paralysis or underventilation due to cephalad extension of the motor level of anesthesia may occur. This may lead to secondary cardiac arrest if untreated. Patients over 65 years, particularly those with hypertension, may be at increased risk for experiencing the hypotensive effects of MARCAINE. Factors influencing plasma protein binding, such as acidosis, systemic diseases which alter protein production, or competition of other drugs for protein binding sites, may diminish individual tolerance.
CNS Reactions: These are characterized by excitation and/or depression. Restlessness, anxiety, dizziness, tinnitus, blurred vision, or tremors may occur, possibly proceeding to convulsions. However, excitement may be transient or absent, with depression being the first manifestation of an adverse reaction. This may quickly be followed by drowsiness merging into unconsciousness and respiratory arrest. Other CNS effects may be nausea, vomiting, chills, and constriction of the pupils.
The incidence of convulsions associated with the use of local anesthetics varies with the procedure used and the total dose administered. In a survey of studies of epidural anesthesia, overt toxicity progressing to convulsions occurred in approximately 0.1% of local anesthetic administrations.
Cardiovascular System Reactions: High doses or unintentional intravascular injection may lead to high plasma levels and related depression of the myocardium, decreased cardiac output, heartblock, hypotension, bradycardia, ventricular arrhythmias, including ventricular tachycardia and ventricular fibrillation, and cardiac arrest (See WARNINGS, PRECAUTIONS, and OVERDOSAGE).
Allergic: Allergic-type reactions are rare and may occur as a result of sensitivity to the local anesthetic or to other formulation ingredients, such as the antimicrobial preservative methylparaben contained in multiple-dose vials or sulfites in epinephrine-containing solutions. These reactions are characterized by signs such as urticaria, pruritus, erythema, angioneurotic edema (including laryngeal edema), tachycardia, sneezing, nausea, vomiting, dizziness, syncope, excessive sweating, elevated temperature, and possibly, anaphylactoid-like symptomatology (including severe hypotension). Cross sensitivity among members of the amide-type local anesthetic group has been reported. The usefulness of screening for sensitivity has not been definitely established.
Neurologic: The incidences of adverse neurologic reactions associated with the use of local anesthetics may be related to the total dose of local anesthetic administered and are also dependent upon the particular drug used, the route of administration, and the physical status of the patient. Many of these effects may be related to local anesthetic techniques, with or without a contribution from the drug.
In the practice of caudal or lumbar epidural block, occasional unintentional penetration of the subarachnoid space by the catheter or needle may occur. Subsequent adverse effects may depend partially on the amount of drug administered intrathecally and the physiological and physical effects of a dural puncture. A high spinal is characterized by paralysis of the legs, loss of consciousness, respiratory paralysis, and bradycardia.
Neurologic effects following epidural or caudal anesthesia may include spinal block of varying magnitude (including high or total spinal block); hypotension secondary to spinal block; urinary retention; fecal and urinary incontinence; loss of perineal sensation and sexual function; persistent anesthesia, paresthesia, weakness, paralysis of the lower extremities and loss of sphincter control all of which may have slow, incomplete, or no recovery; headache; backache; septic meningitis; meningismus; slowing of labor; increased incidence of forceps delivery; and cranial nerve palsies due to traction on nerves from loss of cerebrospinal fluid.
Neurologic effects following other procedures or routes of administration may include persistent anesthesia, paresthesia, weakness, paralysis, all of which may have slow, incomplete, or no recovery.
Storage Conditions
Expiration: see expiry date shown on the package.
The indicated expiry date refers to the product in intact packaging, properly stored.
Do not store above 25°C.
CAUTION: Do not use the medicinal product after the expiry date indicated on the package.
KEEP IT OUT OF THE REACH OF CHILDREN
These solutions are not for spinal anesthesia.
Store at 20 to 25°C (68 to 77°F); excursions permitted between 15° to 30°C (59° to 86°F). [See USP Controlled Room Temperature.]
MARCAINE―Solutions of MARCAINE that do not contain epinephrine may be autoclaved. Autoclave at 15-pound pressure, 121°C (250°F) for 15 minutes.
For single-dose vials: Discard unused portion.
How Supplied
0.25% (2.5 mg/mL, 5 mL and 10 mL single-dose ampules)
0.5% (5 mg/mL, 5 mL and 10 mL single-dose ampules)
0.25% (2.5 mg/mL, 10 mL single-dose vials)
0.5% (5 mg/mL, 10 mL single-dose vials)
-
HOW SUPPLIED
0.25% contains 2.5 mg bupivacaine hydrochloride per mL
NDC 59923-717-05 5 mL single-dose vials (12.5 mg/5 mL) 10 ampules per carton
NDC 59923-719-10 10 mL single-dose vials (25 mg/10 mL) 10 ampules per carton
0.5% contains 5 mg bupivacaine hydrochloride per mL
NDC 59923-718-05 5 mL single-dose vials (25 mg/5 mL) 10 ampules per carton
NDC 59923-720-10 10 mL single-dose vials (50 mg/10 mL) 10 ampules per carton
Store at 20 to 25°C (68 to 77°F). [See USP Controlled Room Temperature.]
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
BUPIVACAINE HYDROCHLORIDE
bupivacaine hydrochloride injectionProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:59923-719 Route of Administration EPIDURAL, INTRACAUDAL, PERINEURAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength BUPIVACAINE HYDROCHLORIDE ANHYDROUS (UNII: AKA908P8J1) (BUPIVACAINE - UNII:Y8335394RO) BUPIVACAINE HYDROCHLORIDE ANHYDROUS 2.5 mg in 1 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) SODIUM CHLORIDE (UNII: 451W47IQ8X) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:59923-719-10 10 in 1 CARTON 07/12/2019 1 10 mL in 1 AMPULE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date Unapproved drug for use in drug shortage 07/12/2019 BUPIVACAINE HYDROCHLORIDE
bupivacaine hydrochloride injectionProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:59923-717 Route of Administration EPIDURAL, INTRACAUDAL, PERINEURAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength BUPIVACAINE HYDROCHLORIDE ANHYDROUS (UNII: AKA908P8J1) (BUPIVACAINE - UNII:Y8335394RO) BUPIVACAINE HYDROCHLORIDE ANHYDROUS 2.5 mg in 1 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) SODIUM CHLORIDE (UNII: 451W47IQ8X) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:59923-717-05 10 in 1 CARTON 07/12/2019 1 5 mL in 1 AMPULE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date Unapproved drug for use in drug shortage 07/12/2019 BUPIVACAINE HYDROCHLORIDE
bupivacaine hydrochloride injectionProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:59923-718 Route of Administration EPIDURAL, INTRACAUDAL, PERINEURAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength BUPIVACAINE HYDROCHLORIDE ANHYDROUS (UNII: AKA908P8J1) (BUPIVACAINE - UNII:Y8335394RO) BUPIVACAINE HYDROCHLORIDE ANHYDROUS 5 mg in 1 mL Inactive Ingredients Ingredient Name Strength SODIUM CHLORIDE (UNII: 451W47IQ8X) WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:59923-718-05 10 in 1 CARTON 07/12/2019 1 5 mL in 1 AMPULE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date Unapproved drug for use in drug shortage 07/12/2019 BUPIVACAINE HYDROCHLORIDE
bupivacaine hydrochloride injectionProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:59923-720 Route of Administration EPIDURAL, INTRACAUDAL, PERINEURAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength BUPIVACAINE HYDROCHLORIDE ANHYDROUS (UNII: AKA908P8J1) (BUPIVACAINE - UNII:Y8335394RO) BUPIVACAINE HYDROCHLORIDE ANHYDROUS 5 mg in 1 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) SODIUM CHLORIDE (UNII: 451W47IQ8X) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:59923-720-10 10 in 1 CARTON 07/12/2019 1 10 mL in 1 AMPULE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date Unapproved drug for use in drug shortage 07/12/2019 Labeler - Areva Pharmaceuticals (833189835) Establishment Name Address ID/FEI Business Operations Fisiopharma SRL 441067444 manufacture(59923-717, 59923-718, 59923-719, 59923-720)
















