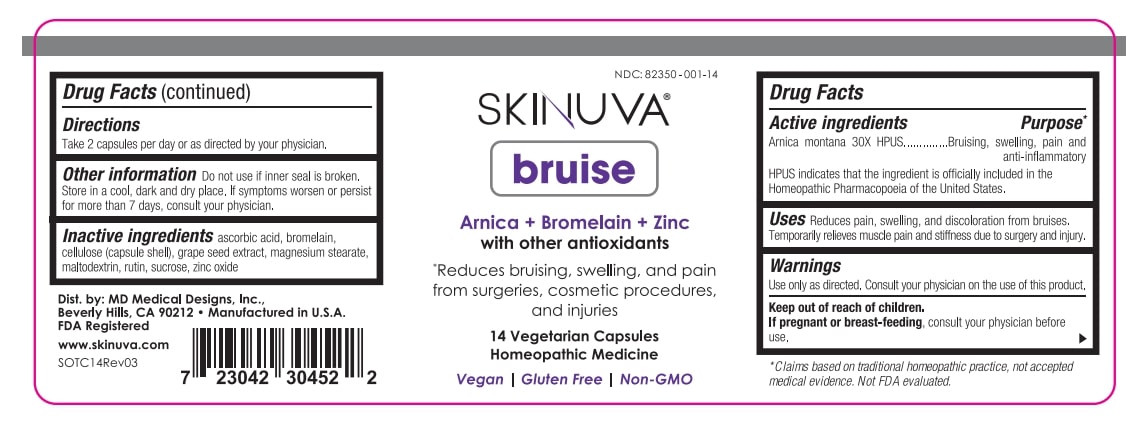
Label: SKINUVA BRUISE- arnica montana, bromelain, grape seed extract, vitamin c, rutin, zinc oxide, cellulose capsule
- NDC Code(s): 82350-001-14
- Packager: MD Medical Designs, Inc.
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: unapproved homeopathic
DISCLAIMER: This homeopathic product has not been evaluated by the Food and Drug Administration for safety or efficacy. FDA is not aware of scientific evidence to support homeopathy as effective.
Drug Label Information
Updated April 10, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
ACTIVE INGREDIENT
Active ingredients Purpose*
Arnica montana 30X HPUS..............Bruising, swelling, pain and
anti-inflammatory
HPUS indicates that the ingredient is officially included in the
Homeopathic Pharmacopoeia of the United States
*Claims based on traditional homeopathic practice, not accepted
medical evidence. Not FDA evaluated.
- INDICATIONS & USAGE
- WARNINGS
- DOSAGE & ADMINISTRATION
- OTHER SAFETY INFORMATION
- INACTIVE INGREDIENT
- QUESTIONS
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
SKINUVA BRUISE
arnica montana, bromelain, grape seed extract, vitamin c, rutin, zinc oxide, cellulose capsuleProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:82350-001 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ARNICA MONTANA (UNII: O80TY208ZW) (ARNICA MONTANA - UNII:O80TY208ZW) ARNICA MONTANA 30 [hp_X] Inactive Ingredients Ingredient Name Strength SUCROSE (UNII: C151H8M554) MAGNESIUM STEARATE (UNII: 70097M6I30) MALTODEXTRIN (UNII: 7CVR7L4A2D) BROMELAINS (UNII: U182GP2CF3) VITIS VINIFERA SEED (UNII: C34U15ICXA) ASCORBIC ACID (UNII: PQ6CK8PD0R) ZINC OXIDE (UNII: SOI2LOH54Z) POWDERED CELLULOSE (UNII: SMD1X3XO9M) RUTIN (UNII: 5G06TVY3R7) Product Characteristics Color gray Score no score Shape capsule Size 23mm Flavor Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:82350-001-14 14 in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product 04/10/2023 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved homeopathic 04/10/2023 Labeler - MD Medical Designs, Inc. (094586004)