Label: TECNU CALAGEL- diphenhydramine hydrochloride gel
- NDC Code(s): 51879-802-06, 51879-802-16, 51879-802-44, 51879-802-66
- Packager: Tec Laboratories Inc.
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated October 2, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- ACTIVE INGREDIENT
- PURPOSE
- INDICATIONS & USAGE
- WARNINGS
- ASK DOCTOR
- KEEP OUT OF REACH OF CHILDREN
- STOP USE
-
DOSAGE & ADMINISTRATION
Directions •do not use more often than directed
•adults and children 2 years of age and older
•cleanse skin with soap and warm water and dry affected area
•apply to affected area not more than 3 times daily
•may be covered with a sterile bandage, if bandaged, let dry first
•children under 2 years of age do not use, consult a doctor
- STORAGE AND HANDLING
- INACTIVE INGREDIENT
- QUESTIONS
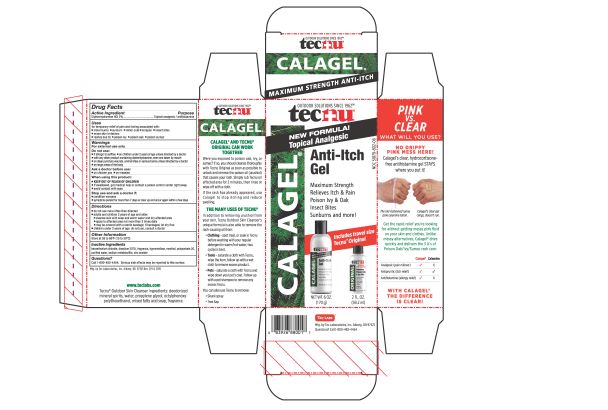
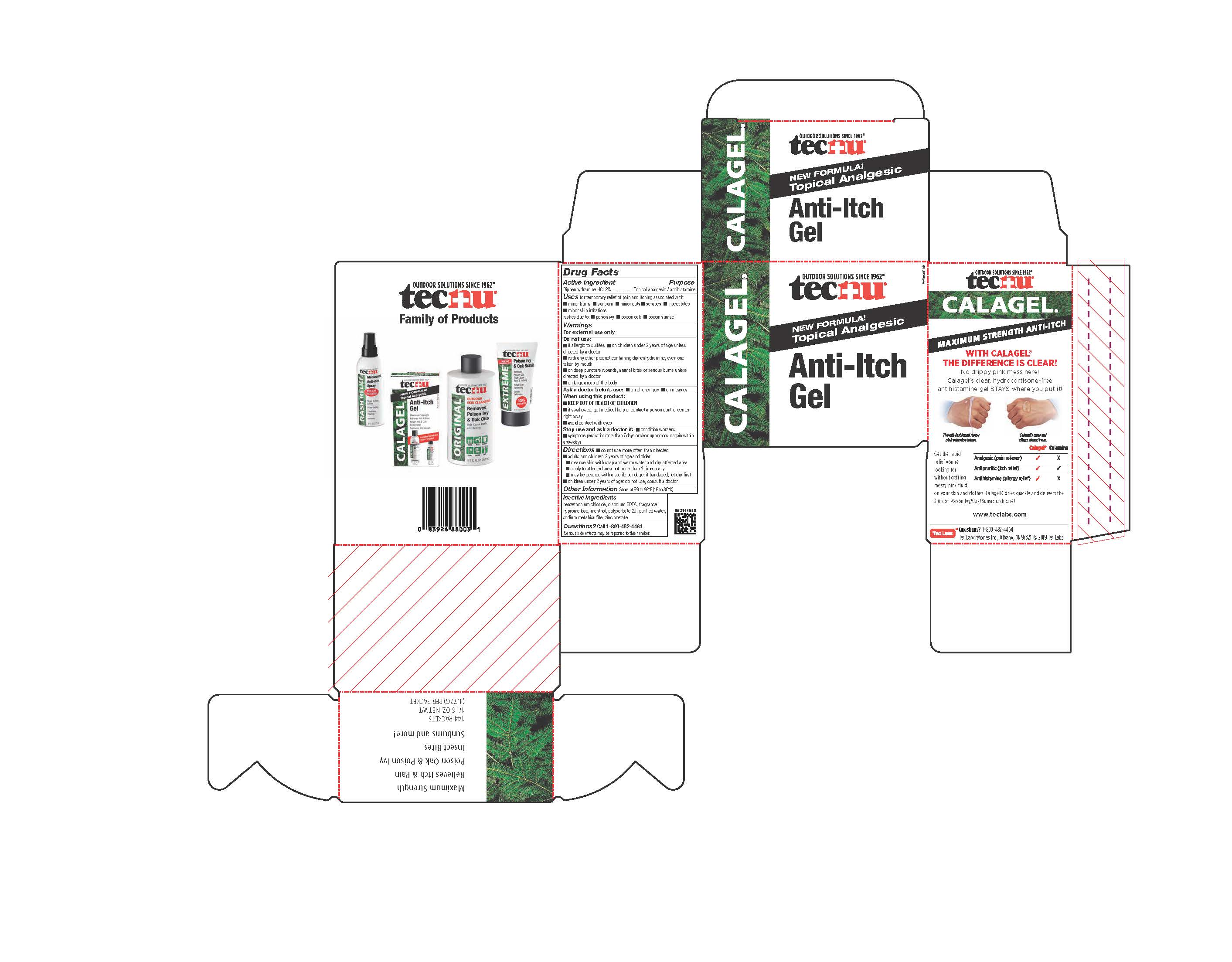
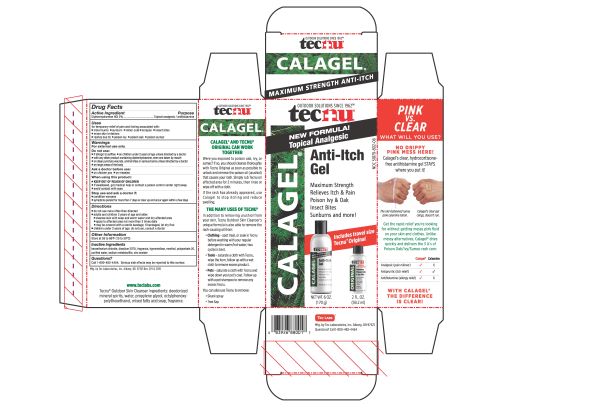
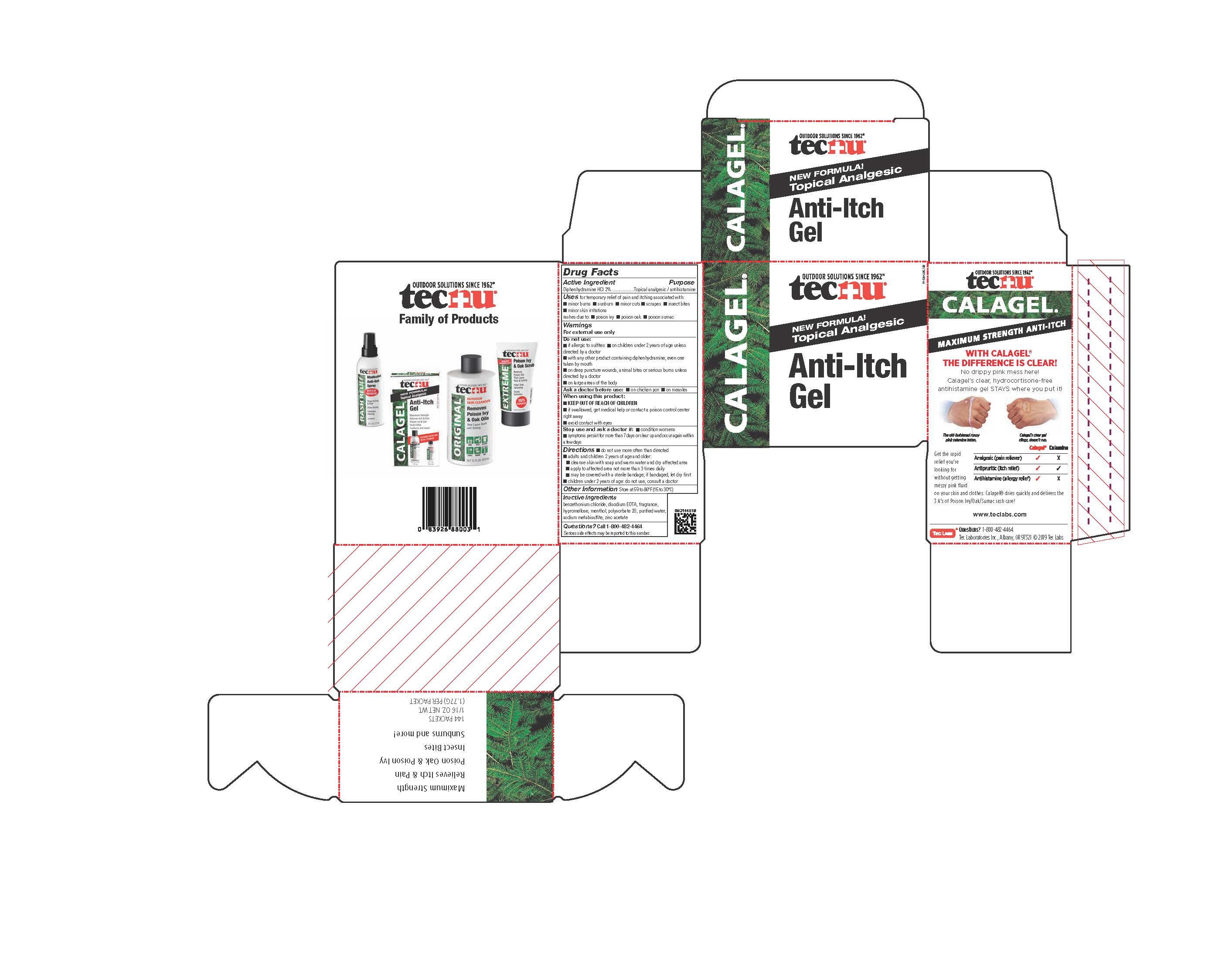
- PRINCIPAL DISPLAY PANEL
- PRINCIPAL DISPLAY PANEL
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
TECNU CALAGEL
diphenhydramine hydrochloride gelProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:51879-802 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength DIPHENHYDRAMINE HYDROCHLORIDE (UNII: TC2D6JAD40) (DIPHENHYDRAMINE - UNII:8GTS82S83M) DIPHENHYDRAMINE HYDROCHLORIDE 20 mg in 1 g Inactive Ingredients Ingredient Name Strength POLYSORBATE 20 (UNII: 7T1F30V5YH) ZINC ACETATE (UNII: FM5526K07A) HYPROMELLOSE 2208 (100 MPA.S) (UNII: B1QE5P712K) WATER (UNII: 059QF0KO0R) MENTHOL, UNSPECIFIED FORM (UNII: L7T10EIP3A) BENZETHONIUM CHLORIDE (UNII: PH41D05744) SODIUM METABISULFITE (UNII: 4VON5FNS3C) EDETATE DISODIUM (UNII: 7FLD91C86K) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:51879-802-06 1 in 1 CARTON 06/20/2019 1 NDC:51879-802-66 178.9 g in 1 BOTTLE; Type 0: Not a Combination Product 2 NDC:51879-802-66 178.9 g in 1 BOTTLE; Type 0: Not a Combination Product 06/20/2019 3 NDC:51879-802-44 144 in 1 CARTON 07/23/2019 3 NDC:51879-802-16 1.86 g in 1 PACKET; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M017 06/20/2019 Labeler - Tec Laboratories Inc. (083647792) Establishment Name Address ID/FEI Business Operations Tec Laboratories Inc. 083647792 manufacture(51879-802)