Label: ECO BARRIER NON CHEMICAL SUN- zinc oxide, titanium dioxide cream
-
Contains inactivated NDC Code(s)
NDC Code(s): 71982-010-01, 71982-010-02 - Packager: JEIL H&B INC.
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: OTC monograph not final
DISCLAIMER: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
Drug Label Information
Updated January 10, 2018
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- ACTIVE INGREDIENT
-
INACTIVE INGREDIENT
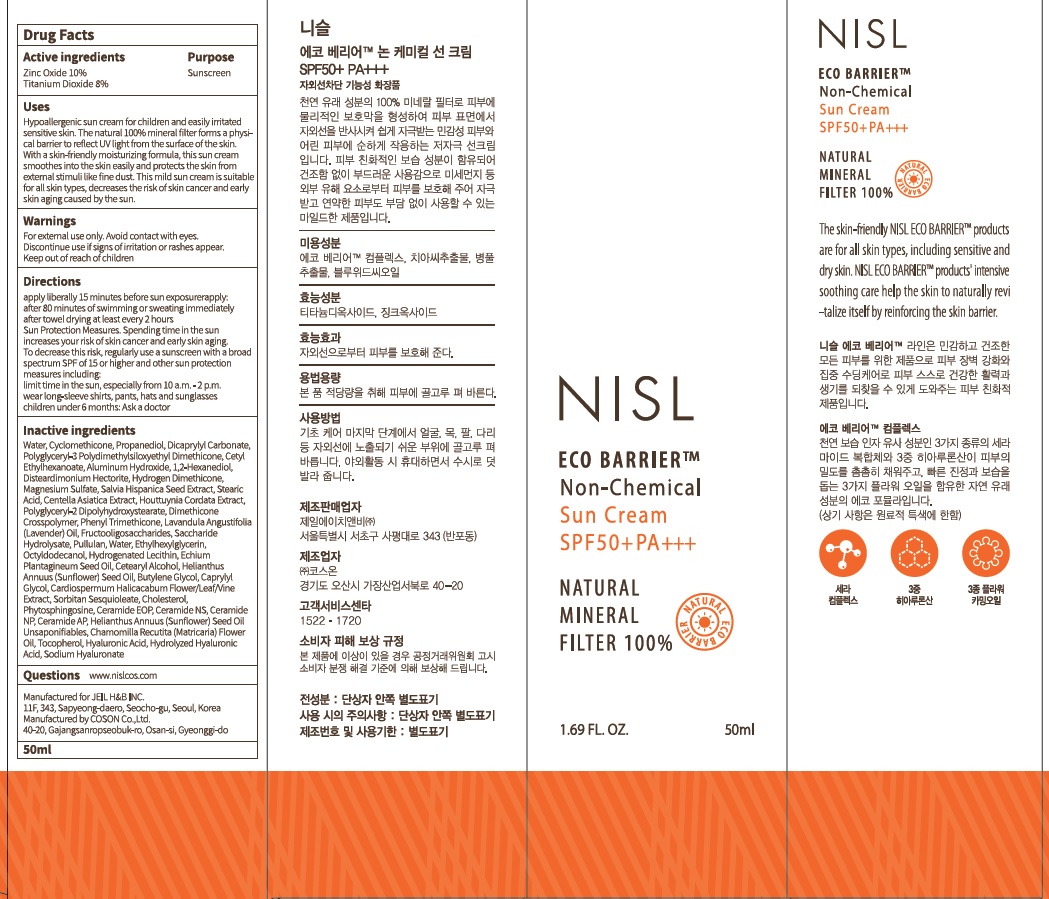
Inactive ingredients: Water, Cyclomethicone, Propanediol, Dicaprylyl Carbonate, Polyglyceryl-3 Polydimethylsiloxyethyl Dimethicone, Cetyl Ethylhexanoate, Aluminum Hydroxide, 1,2-Hexanediol, Disteardimonium Hectorite, Hydrogen Dimethicone, Magnesium Sulfate, Salvia Hispanica Seed Extract, Stearic Acid, Centella Asiatica Extract, Houttuynia Cordata Extract, Polyglyceryl-2 Dipolyhydroxystearate, Dimethicone Crosspolymer, Phenyl Trimethicone, Lavandula Angustifolia (Lavender) Oil, Fructooligosaccharides, Saccharide Hydrolysate, Pullulan, Water, Ethylhexylglycerin, Octyldodecanol, Hydrogenated Lecithin, Echium Plantagineum Seed Oil, Cetearyl Alcohol, Helianthus Annuus (Sunflower) Seed Oil, Butylene Glycol, Caprylyl Glycol, Cardiospermum Halicacabum Flower/Leaf/Vine Extract, Sorbitan Sesquioleate, Cholesterol, Phytosphingosine, Ceramide EOP, Ceramide NS, Ceramide NP, Ceramide AP, Helianthus Annuus (Sunflower) Seed Oil Unsaponifiables, Chamomilla Recutita (Matricaria) Flower Oil, Tocopherol, Hyaluronic Acid, Hydrolyzed Hyaluronic Acid, Sodium Hyaluronate
- PURPOSE
- WARNINGS
-
DESCRIPTION
Uses: Hypoallergenic sun cream for children and easily irritated sensitive skin. The natural 100% mineral filter forms a physical barrier to reflect UV light from the surface of the skin. With a skin-friendly moisturizing formula, this sun cream smoothes into the skin easily and protects the skin from external stimuli like fine dust. This mild sun cream is suitable for all skin types, decreases the risk of skin cancer and early skin aging caused by the sun.
Directions:
Apply liberally 15 minutes before sun exposure
Reapply: after 80 minutes of swimming or sweating immediately after towel drying at least every 2 hours
Sun Protection Measures. Spending time in the sun increases your risk of skin cancer and early skin aging. To decrease this risk, regularly use a sunscreen with a broad spectrum SPF of 15 or higher and other sun protection measures including: limit time in the sun, especially from 10 a.m. - 2 p.m. wear long-sleeve shirts, pants, hats and sunglasses
Children under 6 months: Ask a doctor
Questions: www.nislcos.com
- PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
ECO BARRIER NON CHEMICAL SUN
zinc oxide, titanium dioxide creamProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:71982-010 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Zinc Oxide (UNII: SOI2LOH54Z) (ZINC CATION - UNII:13S1S8SF37) ZINC CATION 5.0 g in 50 mL Titanium Dioxide (UNII: 15FIX9V2JP) (TITANIUM DIOXIDE - UNII:15FIX9V2JP) Titanium Dioxide 4.0 g in 50 mL Inactive Ingredients Ingredient Name Strength Water (UNII: 059QF0KO0R) Propanediol (UNII: 5965N8W85T) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:71982-010-02 1 in 1 CARTON 12/01/2017 1 NDC:71982-010-01 50 mL in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph not final part352 12/01/2017 Labeler - JEIL H&B INC. (694788123) Registrant - JEIL H&B INC. (694788123) Establishment Name Address ID/FEI Business Operations COSON Co., Ltd._Osan Plant 689847210 manufacture(71982-010)