Label: FAMILY DOLLAR DAYTIME AND NIGHTTIME COMBO PACK COLD AND FLU- acetaminophen, dextromethorphan hbr, phenylephrine hydrochloride, and doxylamine succinate kit
- NDC Code(s): 55319-036-08, 55319-096-08, 55319-808-16
- Packager: FAMILY DOLLAR SERVICES INC
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: OTC monograph final
DISCLAIMER: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
Drug Label Information
Updated September 8, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- SPL UNCLASSIFIED SECTION
- Active ingredients (in each 15 mL)
- Uses
- Warnings
- Directions
- Other information
- Inactive ingredients
- Questions?
- SPL UNCLASSIFIED SECTION
- Active ingredients (in each 30 mL)
- Uses
- Warnings
- Directions
- Other information
- Inactive ingredients
- Questions?
- SPL UNCLASSIFIED SECTION
-
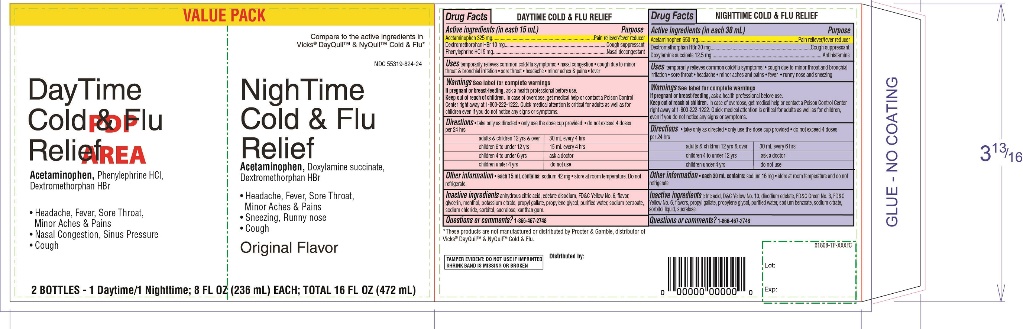
PRINCIPAL DISPLAY PANEL - Kit Carton
Compare to the active ingredients in Vicks®DayQuil™ & NyQuil™ Cold & Flu*
NDC# 55319-808-16
DayTime
Cold & Flu
Relief
Acetaminophen, Dextromethorphan HBr,
Phenylephrine HCl
- •
- Headache, Fever, Sore Throat, Minor Aches & Pains
- •
- Nasal Congestion & Sinus Pressure
- •
- Cough
NightTime
Cold & Flu
Relief
Acetaminophen, Dextromethorphan HBr
Dextromethorphan HBr
- •
- headache, Fever, Sore Throat,
- Minor Aches & Pains
Original Flavor
TAMPER EVIDENT: DO NOT USE IF IMPRINTED SHRINK BAND IS MISSING OR BROKEN.
2 BOTTLES – 1 Daytime/1Nighttime; 8 FL OZ (236 mL) Each; TOTAL 16 FL OZ (472 mL)
*These product is not manufactured or distributed by Procter & Gamble, distributor of Vicks®Dayquil™ & Nyquil™ Cold & flu.
-
INGREDIENTS AND APPEARANCE
FAMILY DOLLAR DAYTIME AND NIGHTTIME COMBO PACK COLD AND FLU
acetaminophen, dextromethorphan hbr, phenylephrine hydrochloride, and doxylamine succinate kitProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:55319-808 Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:55319-808-16 1 in 1 PACKAGE; Type 0: Not a Combination Product 08/21/2023 Quantity of Parts Part # Package Quantity Total Product Quantity Part 1 1 BOTTLE 236 mL Part 2 1 BOTTLE 236 mL Part 1 of 2 FD DAYTIME COLD AND FLU RELIEF
acetaminophen, dextromethorphan hydrobromide, and phenylephrine hydrochloride liquidProduct Information Item Code (Source) NDC:55319-036 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ACETAMINOPHEN (UNII: 362O9ITL9D) (ACETAMINOPHEN - UNII:362O9ITL9D) ACETAMINOPHEN 325 mg in 15 mL DEXTROMETHORPHAN HYDROBROMIDE (UNII: 9D2RTI9KYH) (DEXTROMETHORPHAN - UNII:7355X3ROTS) DEXTROMETHORPHAN HYDROBROMIDE 10 mg in 15 mL PHENYLEPHRINE HYDROCHLORIDE (UNII: 04JA59TNSJ) (PHENYLEPHRINE - UNII:1WS297W6MV) PHENYLEPHRINE HYDROCHLORIDE 5 mg in 15 mL Inactive Ingredients Ingredient Name Strength ANHYDROUS CITRIC ACID (UNII: XF417D3PSL) EDETATE DISODIUM (UNII: 7FLD91C86K) FD&C YELLOW NO. 6 (UNII: H77VEI93A8) GLYCERIN (UNII: PDC6A3C0OX) MENTHOL, UNSPECIFIED FORM (UNII: L7T10EIP3A) POTASSIUM CITRATE (UNII: EE90ONI6FF) PROPYL GALLATE (UNII: 8D4SNN7V92) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) WATER (UNII: 059QF0KO0R) SODIUM BENZOATE (UNII: OJ245FE5EU) SODIUM CHLORIDE (UNII: 451W47IQ8X) SORBITOL (UNII: 506T60A25R) SUCRALOSE (UNII: 96K6UQ3ZD4) XANTHAN GUM (UNII: TTV12P4NEE) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:55319-036-08 236 mL in 1 BOTTLE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph final part341 08/21/2023 Part 2 of 2 FD NIGHTTIME COLD AND FLU RELIEF
acetaminophen, doxylamine succinate, and dextromethorphan hydrobromide liquidProduct Information Item Code (Source) NDC:55319-096 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ACETAMINOPHEN (UNII: 362O9ITL9D) (ACETAMINOPHEN - UNII:362O9ITL9D) ACETAMINOPHEN 650 mg in 30 mL DEXTROMETHORPHAN HYDROBROMIDE (UNII: 9D2RTI9KYH) (DEXTROMETHORPHAN - UNII:7355X3ROTS) DEXTROMETHORPHAN HYDROBROMIDE 30 mg in 30 mL DOXYLAMINE SUCCINATE (UNII: V9BI9B5YI2) (DOXYLAMINE - UNII:95QB77JKPL) DOXYLAMINE SUCCINATE 12.5 mg in 30 mL Inactive Ingredients Ingredient Name Strength ANHYDROUS CITRIC ACID (UNII: XF417D3PSL) D&C YELLOW NO. 10 (UNII: 35SW5USQ3G) EDETATE DISODIUM (UNII: 7FLD91C86K) FD&C GREEN NO. 3 (UNII: 3P3ONR6O1S) FD&C YELLOW NO. 6 (UNII: H77VEI93A8) PROPYL GALLATE (UNII: 8D4SNN7V92) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) WATER (UNII: 059QF0KO0R) SODIUM BENZOATE (UNII: OJ245FE5EU) SODIUM CITRATE, UNSPECIFIED FORM (UNII: 1Q73Q2JULR) SORBITOL (UNII: 506T60A25R) SUCRALOSE (UNII: 96K6UQ3ZD4) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:55319-096-08 236 mL in 1 BOTTLE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph final part341 08/21/2023 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph final part341 08/21/2023 Labeler - FAMILY DOLLAR SERVICES INC (024472631)