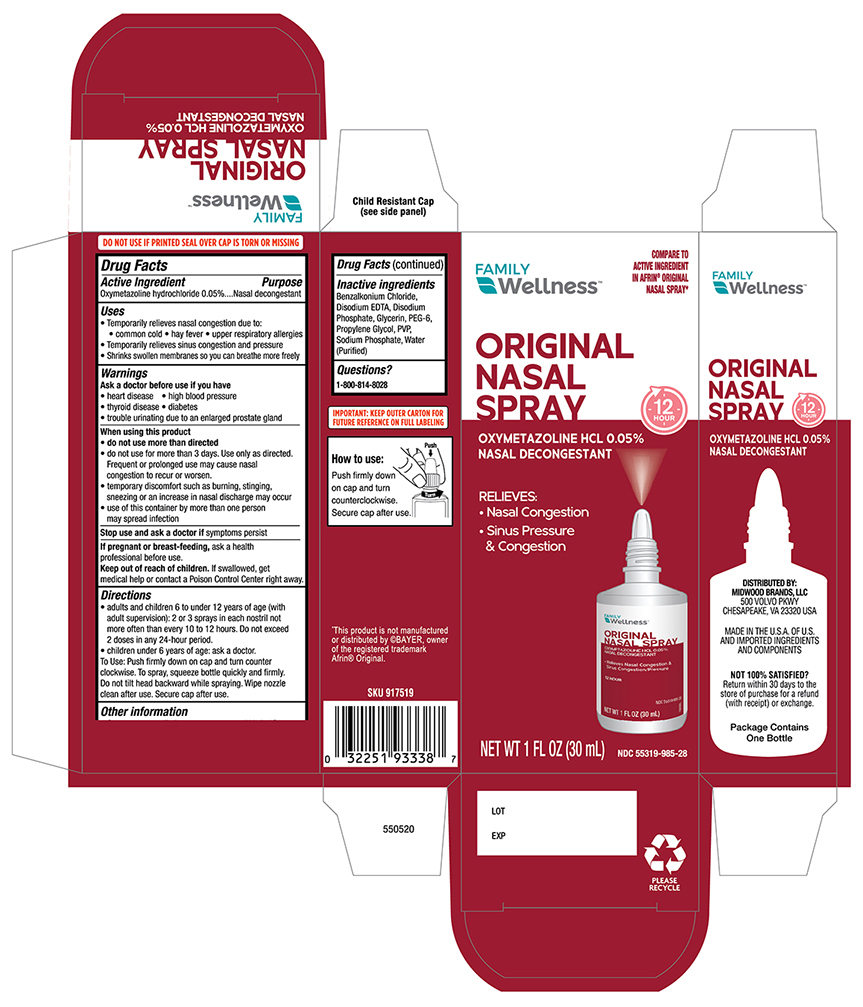
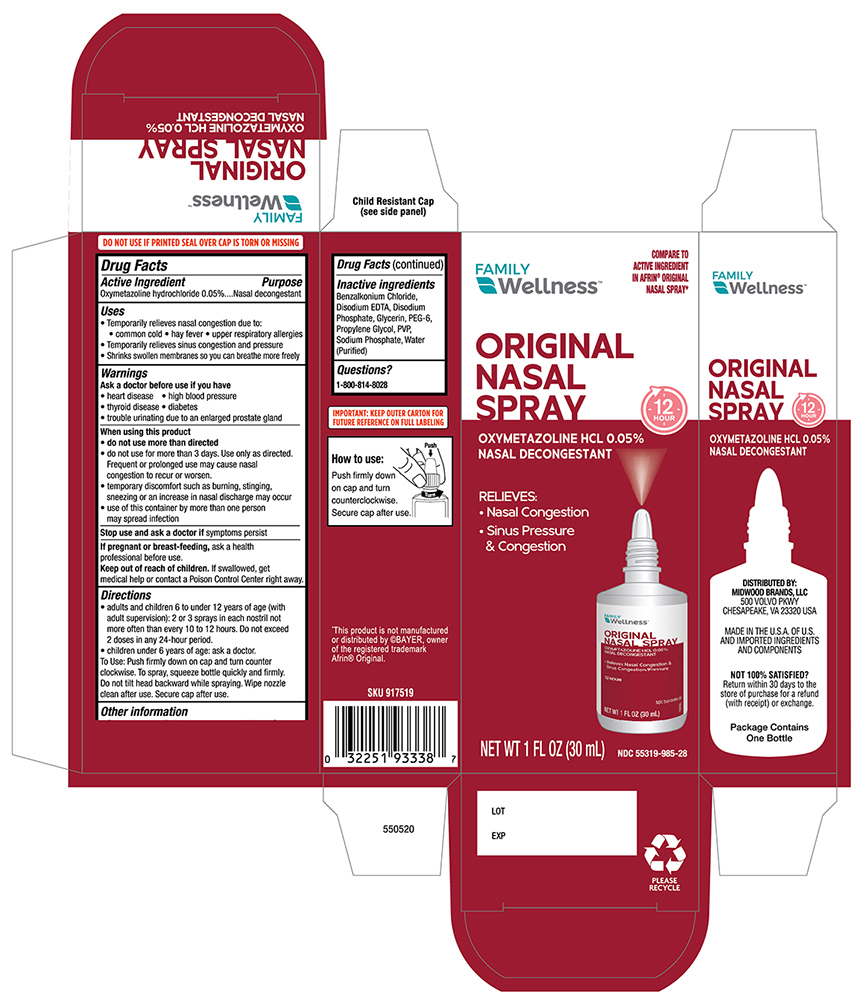
Label: FAMILY WELLNESS OXYMETAZOLINE- oxymetazoline spray
- NDC Code(s): 55319-985-28
- Packager: FAMILY DOLLAR (Family Wellness)
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated December 6, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Drug Fact
- Purpose
- Uses
- Warnings
- Ask a Doctor before use if you have
-
When using this product
Do not use more than directed
- do not use for more than three days, Use only as directed. Frequent or prolonged use may cause nasal congestion to recur or worsen.
- temporary discomfort such as burning, stinging, sneezing or an increase in nasal discharge may occur.
- use of this container by more than one person may spread infection.
- stop use and ask a doctor if
- PREGNANCY OR BREAST FEEDING
- Keep out of the reach of children
-
Directions
- Adults and children 6 to under 12 years of age (with adult supervision):2 or 3 sprays in each nostril not more than every 10 to 12 hours. Do not exceed 2 doses in any 24-hour period.
- Children under 6 years of age consult a doctor.To spray, squeeze bottle quickly and firmly. Do not tilt head backwards while spraying, wipe nozzle clean after use.
- Other Information
- Inactive ingredients:
- Principal Display Panel -Bottle
- Principal Display Panel -Carton
-
INGREDIENTS AND APPEARANCE
FAMILY WELLNESS OXYMETAZOLINE
oxymetazoline sprayProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:55319-985 Route of Administration NASAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength OXYMETAZOLINE HYDROCHLORIDE (UNII: K89MJ0S5VY) (OXYMETAZOLINE - UNII:8VLN5B44ZY) OXYMETAZOLINE HYDROCHLORIDE 50 mg in 100 mL Inactive Ingredients Ingredient Name Strength POLYETHYLENE GLYCOL 300 (UNII: 5655G9Y8AQ) GLYCERIN (UNII: PDC6A3C0OX) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) EDETATE DISODIUM (UNII: 7FLD91C86K) SODIUM PHOSPHATE, MONOBASIC (UNII: 3980JIH2SW) SODIUM PHOSPHATE, DIBASIC, ANHYDROUS (UNII: 22ADO53M6F) POVIDONE (UNII: FZ989GH94E) BENZALKONIUM CHLORIDE (UNII: F5UM2KM3W7) WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:55319-985-28 1 in 1 CARTON 10/02/2023 1 30 mL in 1 BOTTLE, SPRAY; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M012 10/02/2023 Labeler - FAMILY DOLLAR (Family Wellness) (024472631)