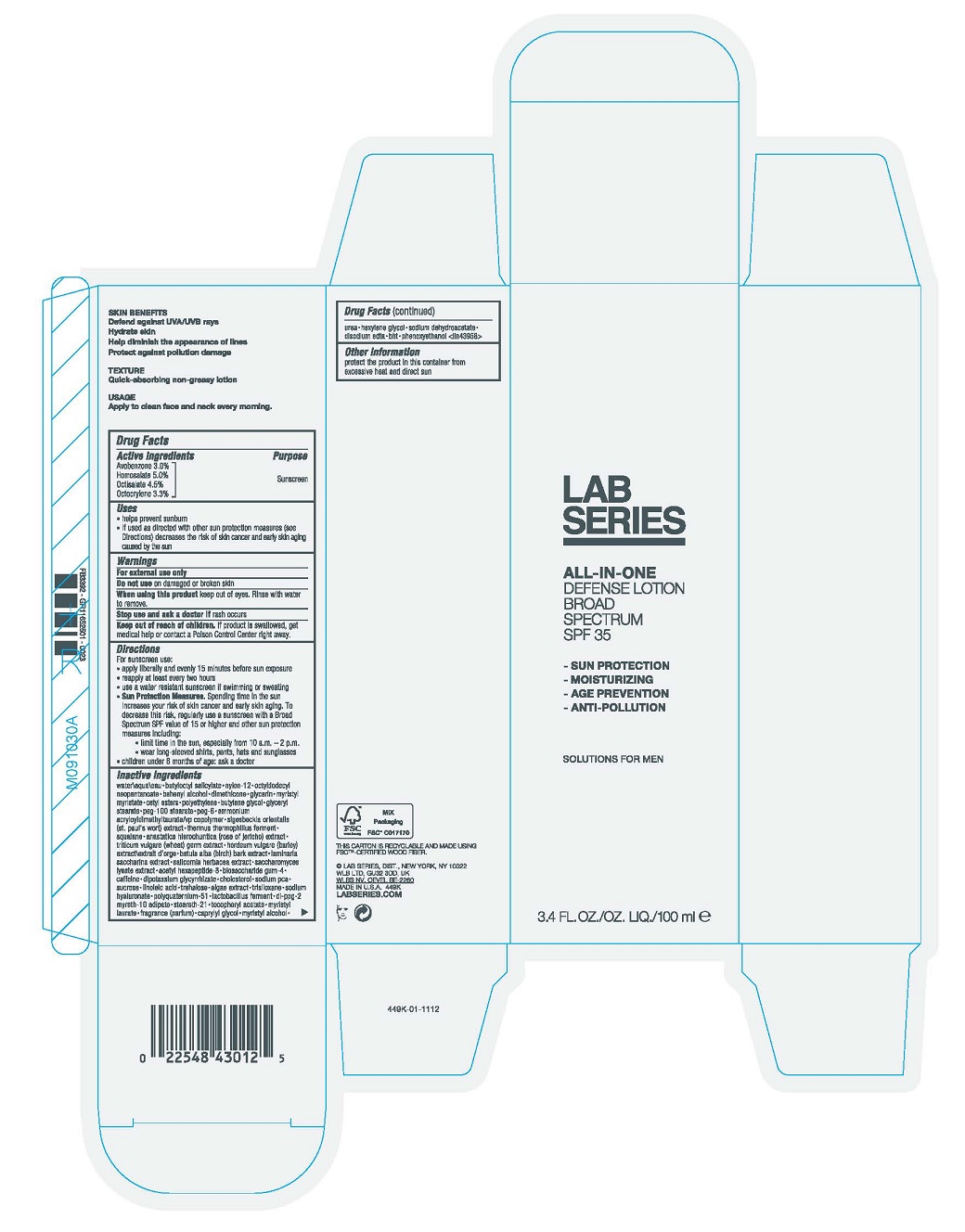
Label: LAB SERIES DAY RESCUE DEFENSE BROAD SPECTRUM SPF 35- avobenzone, homosalate, octisalate, and oxybenzone lotion
- NDC Code(s): 42472-010-01
- Packager: Aramis Inc.
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: OTC monograph final
DISCLAIMER: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
Drug Label Information
Updated May 23, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- SPL UNCLASSIFIED SECTION
- ACTIVE INGREDIENT
-
Uses
- Helps prevent sunburn
- If used as directed with other sun protection measures (see Directions), decreases the risk of skin cancer and early skin aging caused by the sun
- Warnings
-
Directions
- Apply liberally 15 minutes before sun exposure
- Reapply at least every two hours
- Use a water resistant sunscreen if swimming or sweating
-
Sun Protection Measures. Spending time in the sun increases your risk of skin cancer and early skin aging. To decrease this risk, regularly use a sunscreen with a Broad Spectrum SPF value of 15 or higher and other sun protection measures including:
- Limit time in the sun, especially from 10 a.m. – 2 p.m.
- Wear long-sleeved shirts, pants, hats and sunglasses
- Children under 6 months of age: ask a doctor
-
Inactive Ingredients
WATER\AQUA\EAU ∙ BUTYLOCTYL SALICYLATE ∙ NYLON-12 ∙ OCTYLDODECYL NEOPENTANOATE ∙ BEHENYL ALCOHOL ∙ DIMETHICONE ∙ GLYCERIN ∙ MYRISTYL MYRISTATE ∙ CETYL ESTERS ∙ POLYETHYLENE ∙ BUTYLENE GLYCOL ∙ GLYCERYL STEARATE ∙ PEG-100 STEARATE ∙ PEG-6 ∙ AMMONIUM ACRYLOYLDIMETHYLTAURATE/VP COPOLYMER ∙ SIGESBECKIA ORIENTALIS (ST. PAUL'S WORT) EXTRACT ∙ THERMUS THERMOPHILLUS FERMENT ∙ SQUALANE ∙ ANASTATICA HIEROCHUNTICA (ROSE OF JERICHO) EXTRACT ∙ TRITICUM VULGARE (WHEAT) GERM EXTRACT ∙ HORDEUM VULGARE (BARLEY) EXTRACT\EXTRAIT D'ORGE ∙ BETULA ALBA (BIRCH) BARK EXTRACT ∙ LAMINARIA SACCHARINA EXTRACT ∙ SALICORNIA HERBACEA EXTRACT ∙ SACCHAROMYCES LYSATE EXTRACT ∙ ACETYL HEXAPEPTIDE-8 ∙ BIOSACCHARIDE GUM-4 ∙ CAFFEINE ∙ DIPOTASSIUM GLYCYRRHIZATE ∙ CHOLESTEROL ∙ SODIUM PCA ∙ SUCROSE ∙ LINOLEIC ACID ∙ TREHALOSE ∙ ALGAE EXTRACT ∙ TRISILOXANE ∙ SODIUM HYALURONATE ∙ POLYQUATERNIUM-51 ∙ LACTOBACILLUS FERMENT ∙ DI-PPG-2 MYRETH-10 ADIPATE ∙ STEARETH-21 ∙ TOCOPHERYL ACETATE∙ MYRISTYL LAURATE ∙ FRAGRANCE (PARFUM) ∙ CAPRYLYL GLYCOL ∙ MYRISTYL ALCOHOL ∙ UREA ∙ HEXYLENE GLYCOL ∙ SODIUM DEHYDROACETATE ∙ DISODIUM EDTA ∙ BHT ∙ PHENOXYETHANOL <ILN43958>
- Other information
- PRINCIPAL DISPLAY PANEL - 100 ml Tube Carton
-
INGREDIENTS AND APPEARANCE
LAB SERIES DAY RESCUE DEFENSE BROAD SPECTRUM SPF 35
avobenzone, homosalate, octisalate, and oxybenzone lotionProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:42472-010 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength AVOBENZONE (UNII: G63QQF2NOX) (AVOBENZONE - UNII:G63QQF2NOX) AVOBENZONE 0.03 g in 1 mL HOMOSALATE (UNII: V06SV4M95S) (HOMOSALATE - UNII:V06SV4M95S) HOMOSALATE 0.05 g in 1 mL OCTISALATE (UNII: 4X49Y0596W) (OCTISALATE - UNII:4X49Y0596W) OCTISALATE 0.045 g in 1 mL OXYBENZONE (UNII: 95OOS7VE0Y) (OXYBENZONE - UNII:95OOS7VE0Y) OXYBENZONE 0.033 g in 1 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) BUTYLOCTYL SALICYLATE (UNII: 2EH13UN8D3) NYLON-12 (UNII: 446U8J075B) OCTYLDODECYL NEOPENTANOATE (UNII: X8725R883T) DOCOSANOL (UNII: 9G1OE216XY) DIMETHICONE (UNII: 92RU3N3Y1O) GLYCERIN (UNII: PDC6A3C0OX) MYRISTYL MYRISTATE (UNII: 4042ZC00DY) CETYL ESTERS WAX (UNII: D072FFP9GU) HIGH DENSITY POLYETHYLENE (UNII: UG00KM4WR7) BUTYLENE GLYCOL (UNII: 3XUS85K0RA) GLYCERYL MONOSTEARATE (UNII: 230OU9XXE4) PEG-100 STEARATE (UNII: YD01N1999R) POLYETHYLENE GLYCOL 300 (UNII: 5655G9Y8AQ) AMMONIUM ACRYLOYLDIMETHYLTAURATE/VP COPOLYMER (UNII: W59H9296ZG) THERMUS THERMOPHILUS LYSATE (UNII: 775R692494) SQUALANE (UNII: GW89575KF9) SELAGINELLA LEPIDOPHYLLA WHOLE (UNII: 02JQ564P1G) WHEAT (UNII: 4J2I0SN84Y) BARLEY (UNII: 5PWM7YLI7R) ACETYL HEXAPEPTIDE-8 (UNII: L4EL31FWIL) BIOSACCHARIDE GUM-4 (UNII: 9XRL057X90) CAFFEINE (UNII: 3G6A5W338E) GLYCYRRHIZINATE DIPOTASSIUM (UNII: CA2Y0FE3FX) CHOLESTEROL (UNII: 97C5T2UQ7J) SODIUM PYRROLIDONE CARBOXYLATE (UNII: 469OTG57A2) SUCROSE (UNII: C151H8M554) LINOLEIC ACID (UNII: 9KJL21T0QJ) TREHALOSE (UNII: B8WCK70T7I) TRISILOXANE (UNII: 9G1ZW13R0G) HYALURONATE SODIUM (UNII: YSE9PPT4TH) LIMOSILACTOBACILLUS REUTERI (UNII: 9913I24QEE) DI-PPG-2 MYRETH-10 ADIPATE (UNII: 4IN301M0KJ) STEARETH-21 (UNII: 53J3F32P58) MYRISTYL LAURATE (UNII: 58U0NZN2BT) CAPRYLYL GLYCOL (UNII: 00YIU5438U) MYRISTYL ALCOHOL (UNII: V42034O9PU) UREA (UNII: 8W8T17847W) HEXYLENE GLYCOL (UNII: KEH0A3F75J) SODIUM DEHYDROACETATE (UNII: 8W46YN971G) EDETATE DISODIUM ANHYDROUS (UNII: 8NLQ36F6MM) BUTYLATED HYDROXYTOLUENE (UNII: 1P9D0Z171K) PHENOXYETHANOL (UNII: HIE492ZZ3T) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:42472-010-01 1 in 1 CARTON 12/01/2016 1 100 mL in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph final M020 12/01/2016 Labeler - Aramis Inc. (042918826) Registrant - Estee Lauder Companies Inc. (790802086) Establishment Name Address ID/FEI Business Operations The Estee Lauder Inc 802599436 manufacture(42472-010) , pack(42472-010) , label(42472-010)

 LAB
LAB