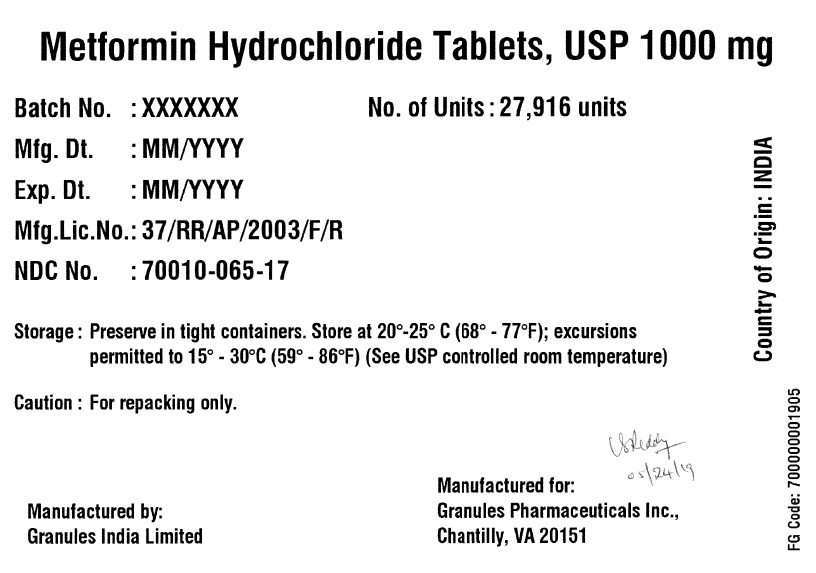
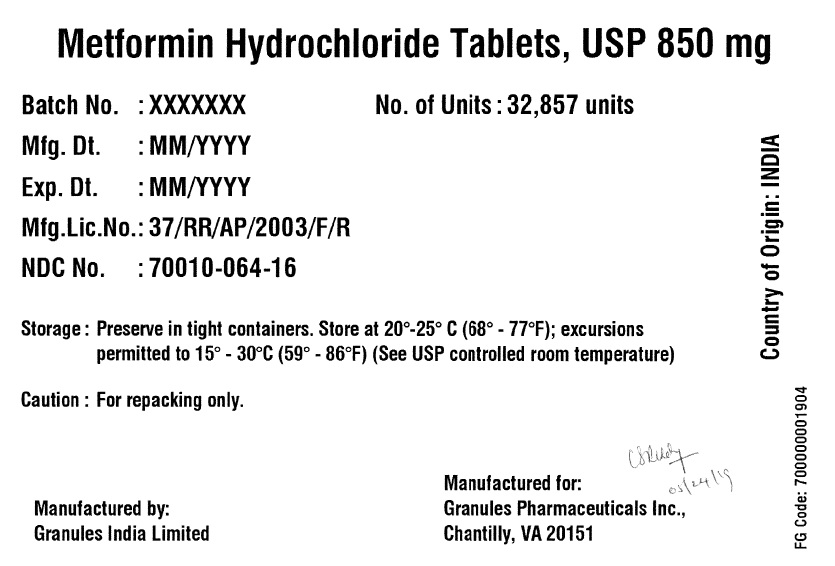
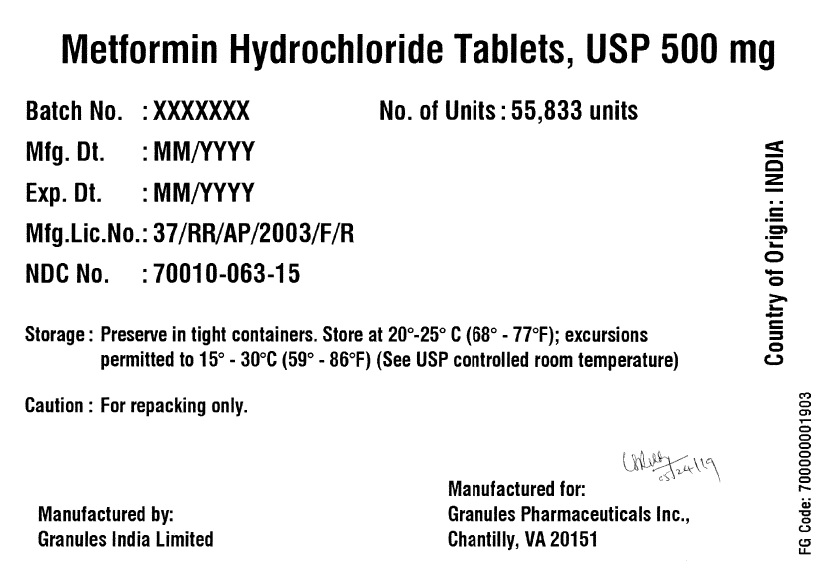
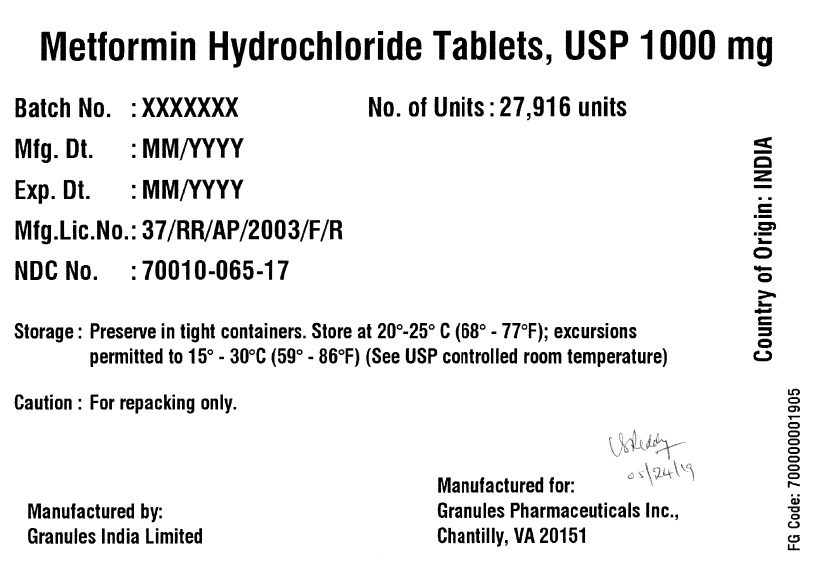
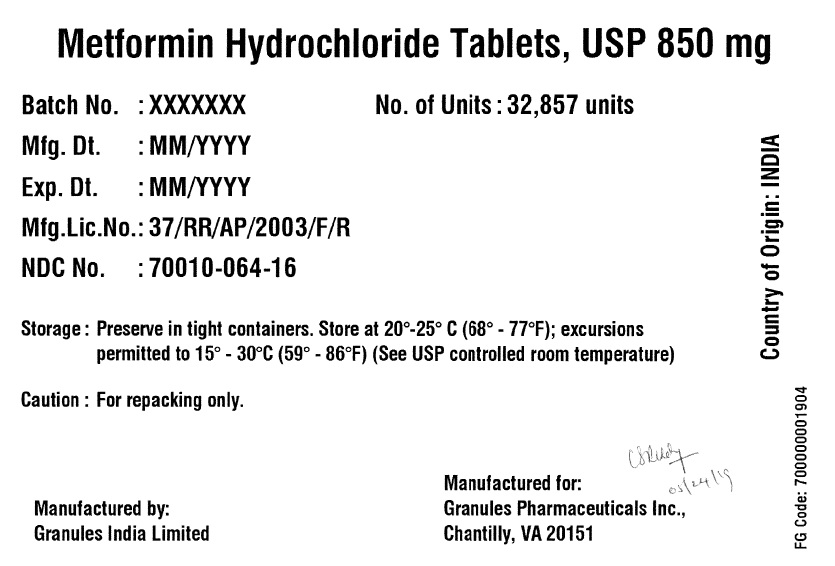
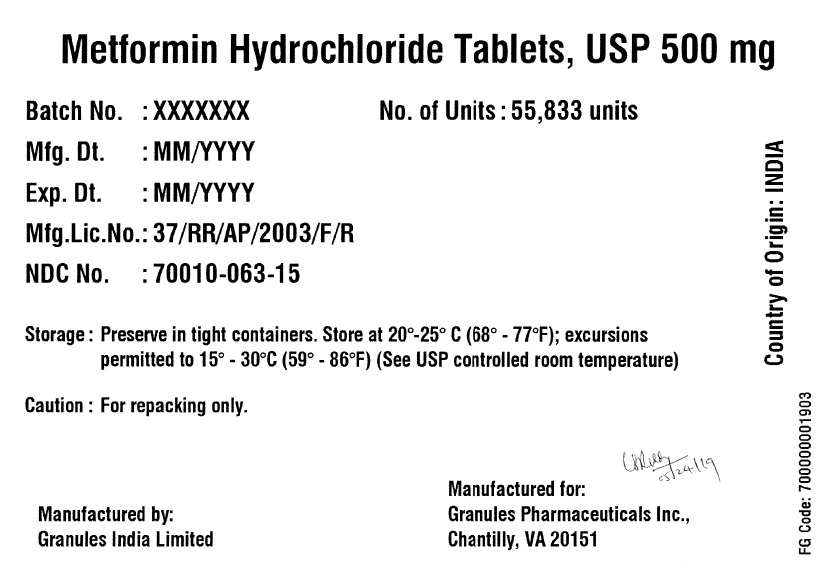
Label: METFORMIN HYDROCHLORIDE tablet
- NDC Code(s): 70010-063-15, 70010-064-16, 70010-065-17
- Packager: Granules Pharmaceuticals Inc
- Category: BULK INGREDIENT
- DEA Schedule: None
- Marketing Status: Export only
Drug Label Information
Updated January 27, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
METFORMIN HYDROCHLORIDE
metformin hydrochloride tabletProduct Information Product Type BULK INGREDIENT Item Code (Source) NDC:70010-063 Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength METFORMIN HYDROCHLORIDE (UNII: 786Z46389E) (METFORMIN - UNII:9100L32L2N) METFORMIN HYDROCHLORIDE 500 mg Product Characteristics Color white (White to off-white) Score no score Shape ROUND (Round biconvex) Size 11mm Flavor BLACKBERRY Imprint Code G;10 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:70010-063-15 55833 in 1 DRUM 06/07/2019 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date Export only 06/07/2019 METFORMIN HYDROCHLORIDE
metformin hydrochloride tabletProduct Information Product Type BULK INGREDIENT Item Code (Source) NDC:70010-064 Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength METFORMIN HYDROCHLORIDE (UNII: 786Z46389E) (METFORMIN - UNII:9100L32L2N) METFORMIN HYDROCHLORIDE 850 mg Product Characteristics Color white (White to off White) Score no score Shape ROUND (Round biconvex) Size 13mm Flavor BLACKBERRY Imprint Code G;11 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:70010-064-16 32857 in 1 DRUM 06/07/2019 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date Export only 06/07/2019 METFORMIN HYDROCHLORIDE
metformin hydrochloride tabletProduct Information Product Type BULK INGREDIENT Item Code (Source) NDC:70010-065 Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength METFORMIN HYDROCHLORIDE (UNII: 786Z46389E) (METFORMIN - UNII:9100L32L2N) METFORMIN HYDROCHLORIDE 1000 mg Product Characteristics Color white (White to off White) Score no score Shape OVAL Size 19mm Flavor BLACKBERRY Imprint Code G;12 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:70010-065-17 27916 in 1 DRUM 06/07/2019 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date Export only 06/07/2019 Labeler - Granules Pharmaceuticals Inc (079825711)