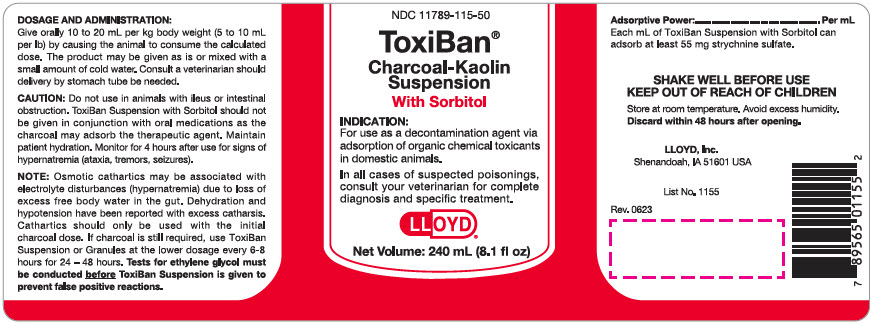
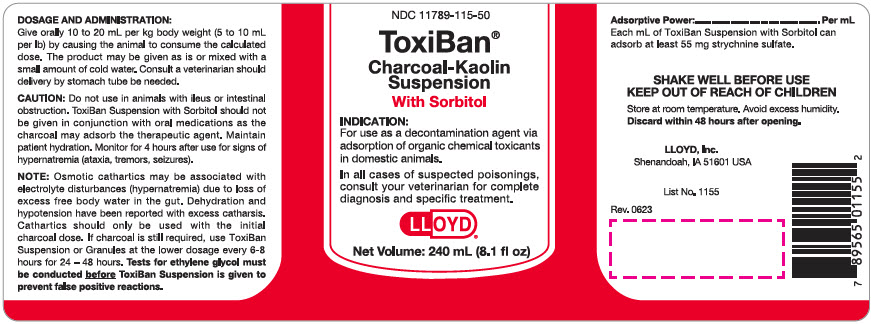
Label: TOXIBAN WITH SORBITOL- activated charcoal suspension
- NDC Code(s): 11789-115-50
- Packager: LLOYD, Inc. of Iowa
- Category: OTC ANIMAL DRUG LABEL
- DEA Schedule: None
- Marketing Status: unapproved drug other
DISCLAIMER: This drug has not been found by FDA to be safe and effective, and this labeling has not been approved by FDA. For further information about unapproved drugs, click here.
Drug Label Information
Updated July 11, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- VETERINARY INDICATIONS
- DOSAGE & ADMINISTRATION
-
PRECAUTIONS
CAUTION: Do not use in animals with ileus or intestinal obstruction. ToxiBan Suspension with Sorbitol should not be given in conjunction with oral medications as the charcoal may adsorb the therapeutic agent. Maintain patient hydration. Monitor for 4 hours after use for signs of hypernatremia (ataxia, tremors, seizures).
NOTE: Osmotic cathartics may be associated with electrolyte disturbances (hypernatremia) due to loss of excess free body water in the gut. Dehydration and hypotension have been reported with excess catharsis. Cathartics should only be used with the initial charcoal dose. If charcoal is still required, use ToxiBan Suspension or Granules at the lower dosage every 6-8 hours for 24 - 48 hours. Tests for ethylene glycol must be conducted before ToxiBan Suspension is given to prevent false positive reactions.
Adsorptive Power:........................ Per ml
Each ml of ToxiBan Suspension with Sorbitol can adsorb at least 55 mg strychnine sulfate.
- STORAGE AND HANDLING
- SPL UNCLASSIFIED SECTION
-
PRINCIPAL DISPLAY PANEL - 240 mL Bottle Label
NDC 11789-115-50
ToxiBan®
Charcoal-Kaolin
Suspension
With SorbitolINDICATION:
For use as a decontamination agent via
adsorption of organic chemical toxicants
in domestic animals.In all cases of suspected poisonings,
consult your veterinarian for complete
diagnosis and specific treatment.LLOYD®
Net Volume: 240 mL (8.1 fl oz)
-
INGREDIENTS AND APPEARANCE
TOXIBAN WITH SORBITOL
activated charcoal suspensionProduct Information Product Type OTC ANIMAL DRUG Item Code (Source) NDC:11789-115 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ACTIVATED CHARCOAL (UNII: 2P3VWU3H10) (ACTIVATED CHARCOAL - UNII:2P3VWU3H10) ACTIVATED CHARCOAL 55 [arb'U] in 1 mL Inactive Ingredients Ingredient Name Strength SORBITOL (UNII: 506T60A25R) KAOLIN (UNII: 24H4NWX5CO) METHYLPARABEN (UNII: A2I8C7HI9T) Product Characteristics Color BLACK (BLACK LIQUID) Score Shape Size Flavor Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:11789-115-50 240 mL in 1 BOTTLE Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date UNAPPROVED DRUG OTHER 07/07/2023 Labeler - LLOYD, Inc. of Iowa (962286535) Registrant - LLOYD, Inc. of Iowa (007281942) Establishment Name Address ID/FEI Business Operations LLOYD, Inc. of Iowa 962286535 LABEL, PACK, API MANUFACTURE, MANUFACTURE Establishment Name Address ID/FEI Business Operations LLOYD, Inc. of Iowa 007281942 ANALYSIS