Label: PEPPLUS SPECIAL SKIN CARE LIFTING PROGRAM- copper tripeptide-1, nicotinoyl dipeptide-230, sh-polypeptide-10, hexapeptide-67 palmitate, camellia sinensis leaf extract kit
- NDC Code(s): 72211-006-01, 72211-007-01, 72211-008-01
- Packager: Picobio Co., Ltd.
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: unapproved drug other
DISCLAIMER: This drug has not been found by FDA to be safe and effective, and this labeling has not been approved by FDA. For further information about unapproved drugs, click here.
Drug Label Information
Updated November 2, 2021
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
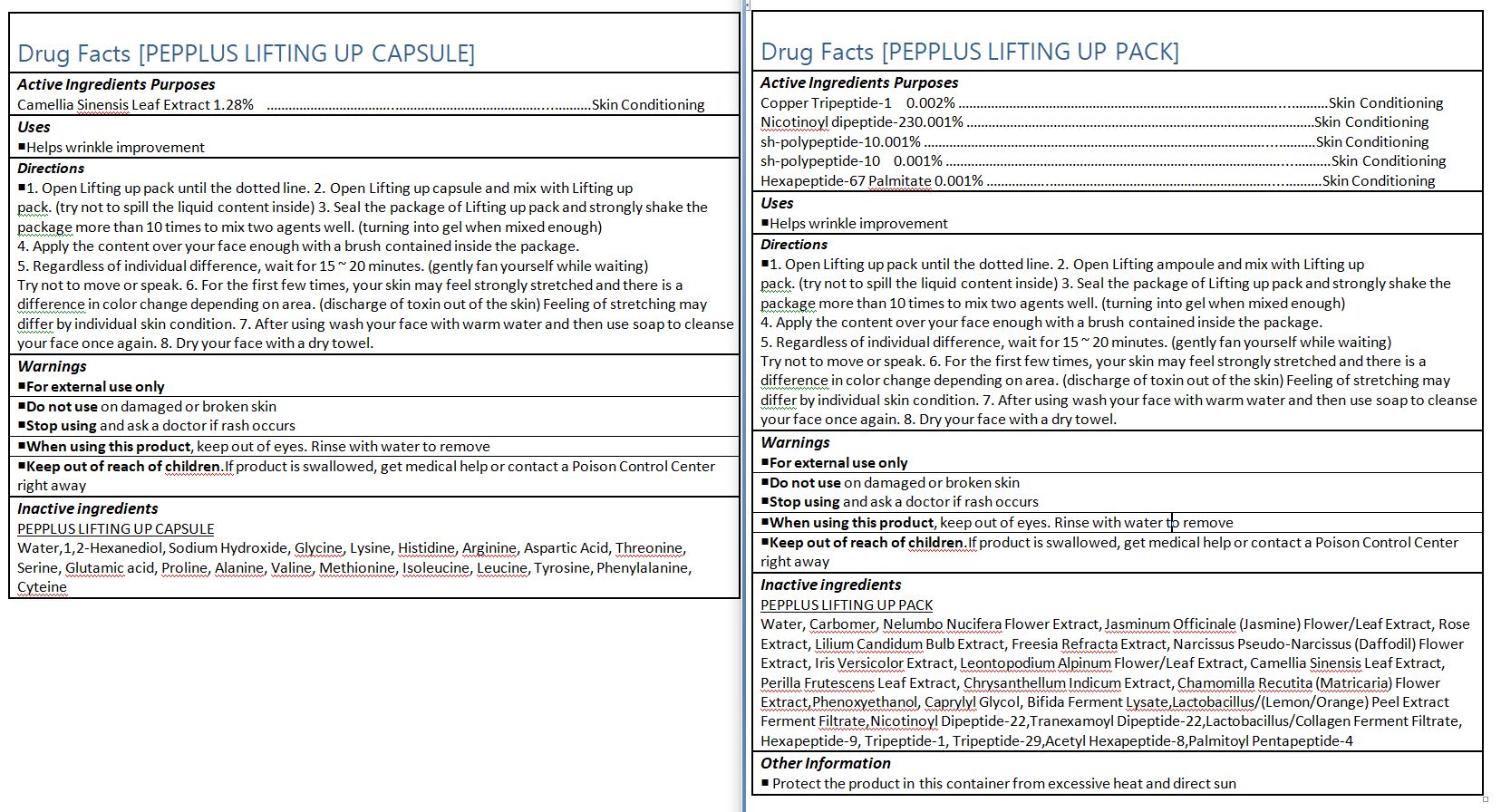
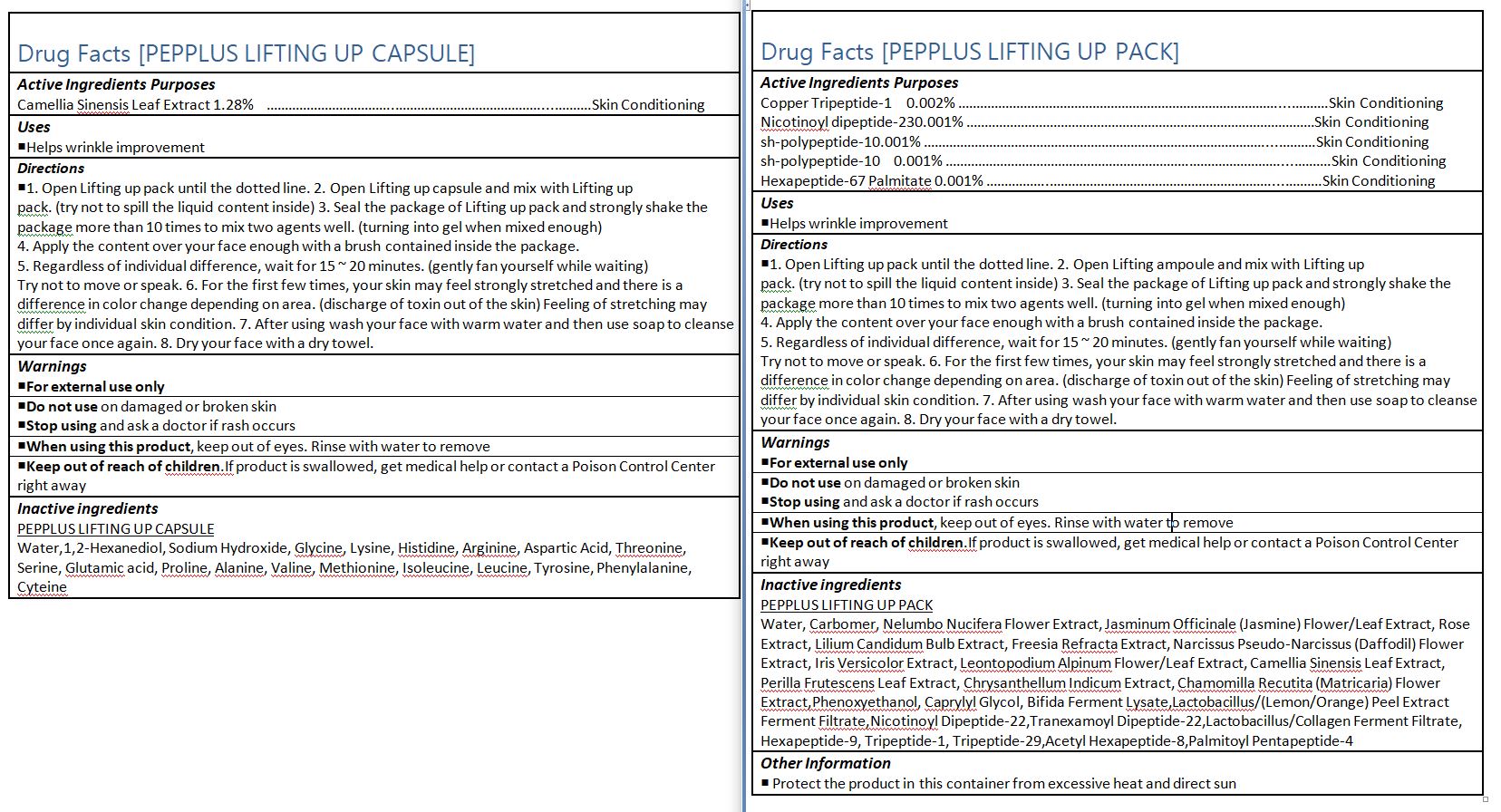
- ACTIVE INGREDIENT
-
INACTIVE INGREDIENT
PEPPLUS LIFTING UP CAPSULE
Water,1,2-Hexanediol, Sodium Hydroxide, Glycine, Lysine, Histidine, Arginine, Aspartic Acid, Threonine, Serine, Glutamic acid, Proline, Alanine, Valine, Methionine, Isoleucine, Leucine, Tyrosine, Phenylalanine, Cyteine
PEPPLUS LIFTING UP PACK
Water, Carbomer, Nelumbo Nucifera Flower Extract, Jasminum Officinale (Jasmine) Flower/Leaf Extract, Rose Extract, Lilium Candidum Bulb Extract, Freesia Refracta Extract, Narcissus Pseudo-Narcissus (Daffodil) Flower Extract, Iris Versicolor Extract, Leontopodium Alpinum Flower/Leaf Extract, Camellia Sinensis Leaf Extract, Perilla Frutescens Leaf Extract, Chrysanthellum Indicum Extract, Chamomilla Recutita (Matricaria) Flower Extract,Phenoxyethanol, Caprylyl Glycol, Bifida Ferment Lysate,Lactobacillus/(Lemon/Orange) Peel Extract Ferment Filtrate,Nicotinoyl Dipeptide-22,Tranexamoyl Dipeptide-22,Lactobacillus/Collagen Ferment Filtrate, Hexapeptide-9, Tripeptide-1, Tripeptide-29,Acetyl Hexapeptide-8,Palmitoyl Pentapeptide-4
- PURPOSE
- KEEP OUT OF REACH OF CHILDREN
-
INDICATIONS & USAGE
1. Open Lifting up pack until the dotted line. 2. Open Lifting up capsule and mix with Lifting up
pack. (try not to spill the liquid content inside) 3. Seal the package of Lifting up pack and strongly shake the
package more than 10 times to mix two agents well. (turning into gel when mixed enough)
4. Apply the content over your face enough with a brush contained inside the package.
5. Regardless of individual difference, wait for 15 ~ 20 minutes. (gently fan yourself while waiting)
Try not to move or speak. 6. For the first few times, your skin may feel strongly stretched and there is a
difference in color change depending on area. (discharge of toxin out of the skin) Feeling of stretching may
differ by individual skin condition. 7. After using wash your face with warm water and then use soap to cleanse your face once again. 8. Dry your face with a dry towel.
-
WARNINGS
1. Do not use in the following cases(Eczema and scalp wounds)
2.Side Effects
1)Due to the use of this druf if rash, irritation, itching and symptopms of hypersnesitivity occur dicontinue use and consult your phamacisr or doctor
3.General Precautions
1)If in contact with the eyes, wash out thoroughty with water If the symptoms are servere, seek medical advice immediately
2)This product is for exeternal use only. Do not use for internal use
4.Storage and handling precautions
1)If possible, avoid direct sunlight and store in cool and area of low humidity
2)In order to maintain the quality of the product and avoid misuse
3)Avoid placing the product near fire and store out in reach of children - DOSAGE & ADMINISTRATION
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
PEPPLUS SPECIAL SKIN CARE LIFTING PROGRAM
copper tripeptide-1, nicotinoyl dipeptide-230, sh-polypeptide-10, hexapeptide-67 palmitate, camellia sinensis leaf extract kitProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:72211-008 Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:72211-008-01 1 in 1 BOX; Type 0: Not a Combination Product 11/02/2021 Quantity of Parts Part # Package Quantity Total Product Quantity Part 1 6 PACKAGE 48 mL Part 2 8 VIAL 8 mL Part 1 of 2 PEPPLUS LIFTING UP PACK
copper tripeptide-1, nicotinoyl dipeptide-230, sh-polypeptide-10, hexapeptide-67 palmitate liquidProduct Information Item Code (Source) NDC:72211-006 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength BASIC FIBROBLAST GROWTH FACTOR (HUMAN) (UNII: S3529G9M9V) (BASIC FIBROBLAST GROWTH FACTOR (HUMAN) - UNII:S3529G9M9V) BASIC FIBROBLAST GROWTH FACTOR (HUMAN) 0.001 g in 100 mL PREZATIDE COPPER (UNII: 6BJQ43T1I9) (PREZATIDE COPPER - UNII:6BJQ43T1I9) PREZATIDE COPPER 0.002 g in 100 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) NELUMBO NUCIFERA FLOWER OIL (UNII: P658Q19EG2) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:72211-006-01 8 mL in 1 PACKAGE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved drug other 11/02/2021 Part 2 of 2 PEPPLUS LIFTING UPCAPSULE
camellia sinensis leaf extract liquidProduct Information Item Code (Source) NDC:72211-007 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength GREEN TEA LEAF (UNII: W2ZU1RY8B0) (GREEN TEA LEAF - UNII:W2ZU1RY8B0) GREEN TEA LEAF 1.28 g in 100 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) 1,2-HEXANEDIOL (UNII: TR046Y3K1G) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:72211-007-01 1 mL in 1 VIAL; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved drug other 11/02/2021 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved drug other 11/02/2021 Labeler - Picobio Co., Ltd. (688821818) Registrant - Picobio Co., Ltd. (688821818) Establishment Name Address ID/FEI Business Operations Picobio Co., Ltd. 688821818 manufacture(72211-008) , label(72211-008)