Label: 4360 FIRST AID KIT kit
-
NDC Code(s):
0498-0100-02,
0498-0143-04,
0498-0221-59,
0498-0402-59, view more0498-0730-01, 0498-0801-35, 0498-4360-01, 59898-420-36
- Packager: Honeywell Safety Products USA, INC
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: unapproved drug other
DISCLAIMER: This drug has not been found by FDA to be safe and effective, and this labeling has not been approved by FDA. For further information about unapproved drugs, click here.
Drug Label Information
Updated January 22, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Eyesaline Active ingredient
- Eyesaline Purpose
- Eyesaline Uses
-
Eyesaline
Warnings
For external use only-
Obtain immediate medical treatment for all open wounds in or near eyes.
To avoid contamination, do not touch tip of container to any surface.
Do not reuse. Once opened, discard.
Do not use
- if solution changes color or becomes cloudy
- if you have open wounds in or near the eyes, get medical help right away.
- Eyesaline Directions
- Eyesaline Inactive ingredients
- Eyesaline Questions
- Alcohol Wipe Active ingredient
- Alcohol Wipe Purpose
- Alcohol Wipe Uses
- Alcohol Wipe Warnings
- Alcohol Wipe Directions
- Alcohol Wipe Other information
- Alcohol Wipe Inactive ingredient
- Alcohol Wipe Questions
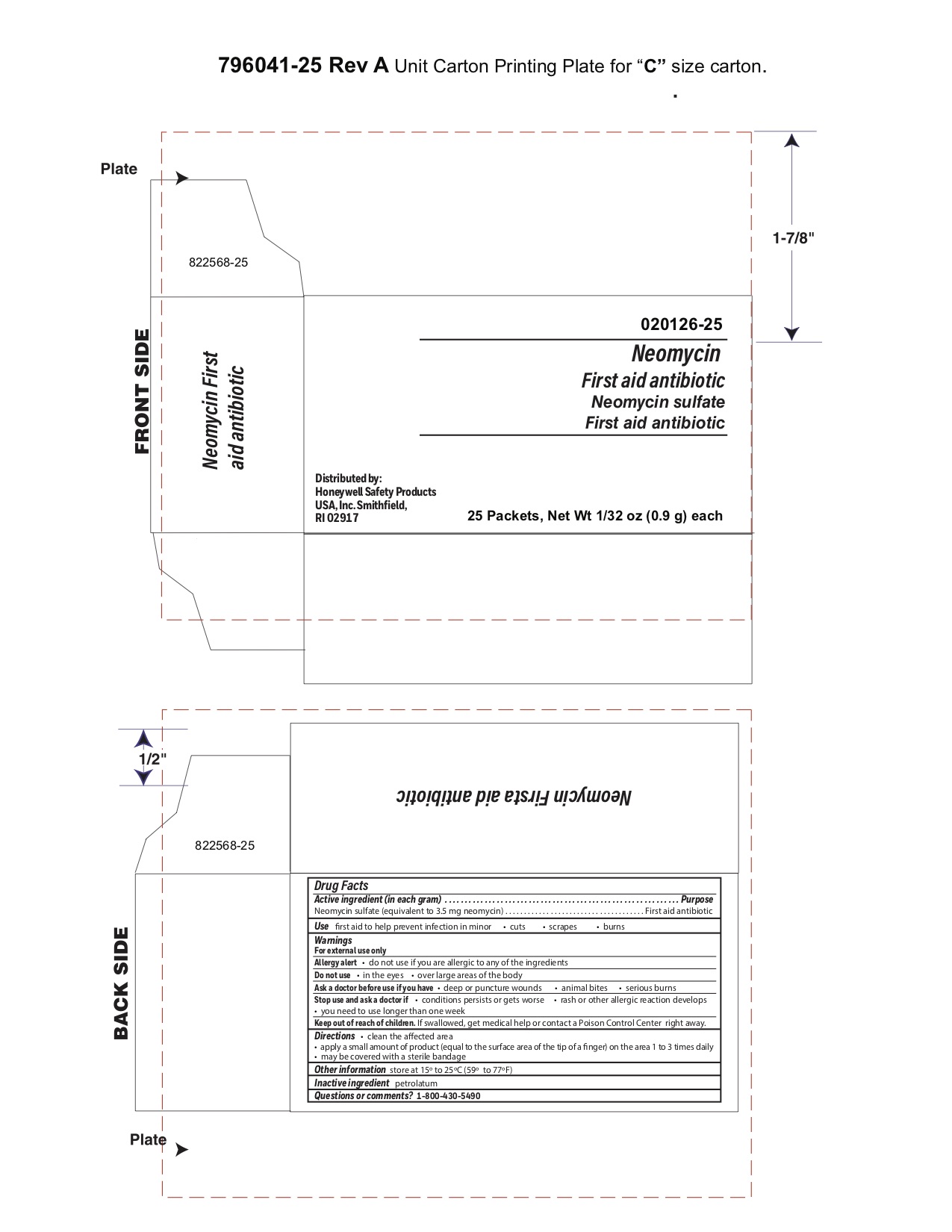
- Neomycin Active ingredient
- Neomycin Purpose
- Neomycin Uses
- Neomycin Warnings
- Neomycin Direction
- Neomycin Other information
- Neomycin Inactive ingredient
- Neomycin Questions?
- Antiseptic Spray Active ingredient
- Antiseptic Spray Purpose
- Antiseptic Spray Uses
- Antiseptic Spray Warnings
- Antiseptic Spray Directions
- Antiseptic Spray Other information
- Antiseptic Spray Inactive ingredients
- Antiseptic Spray Questions
- Burn Spray Active ingredient
- Burn Spray Purpose
- Burn Spray Uses
-
Burn Spray
Warnings
For external use only
Flammable
- keep away from fire or flame
- contents under pressure
- do not puncture or incinerate container
- do not expose to temperatures above 120 0 F
Do not use
- in or near the eyes or other mucous membranes
- in case of serious burns
- in case of deep or puncture wounds
- for prolonged period of time
- on large portion of the body
- Burn Spray Directions
- Burn Spray Other information
- Burn Spray Inactive ingredients
- Hans Sanitizer Active ingredient
- Hand Santizer Purpose
- Hand Sanitizer Uses
- Hand Sanitizer Warnings
- Hand Sanitizer Directions
- Hand Sanitizer Other information
- Hand Sanitizer Inactive ingredients
- Hand Sanitizer Questions or Comments?
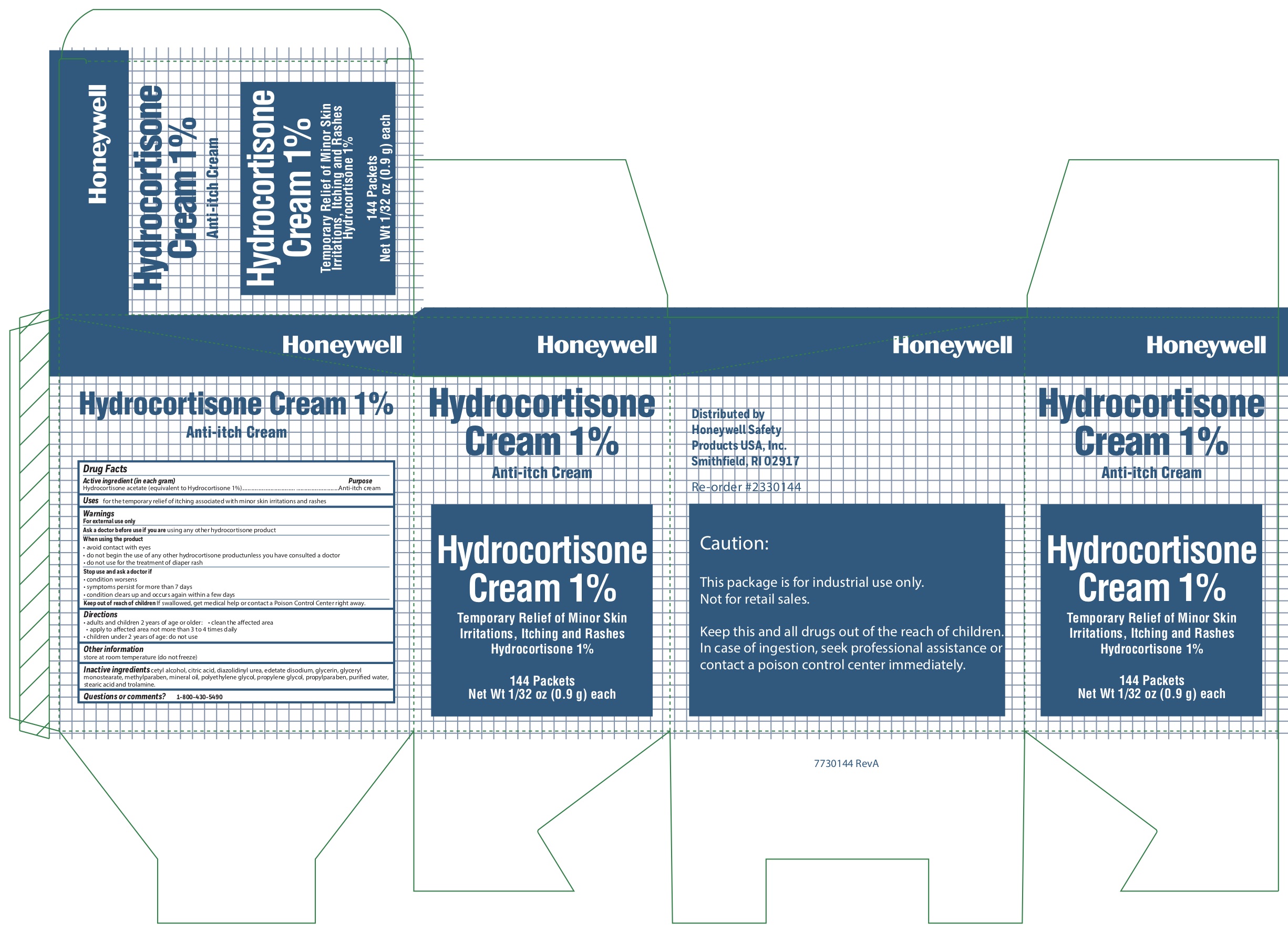
- Hydrocortisone Active ingredient (in each gram)
- Hydrocortisone Purpose
- Hydrocortisone Uses
-
Hydrocortisone
Warnings
For external use ;only
When using the product
- avoid contact with eyes
- do not begin use of any other hydrocortisone product unless you have consulted a doctor
- do not use for the treatment of diaper rash
- Hydrocortisone Directions
- Hydrocortisone Other information
- Hydrocortisone Inactive ingredients
- Hydrocortisone Questions or Comments?
- Eyesaline Principal Display Panel
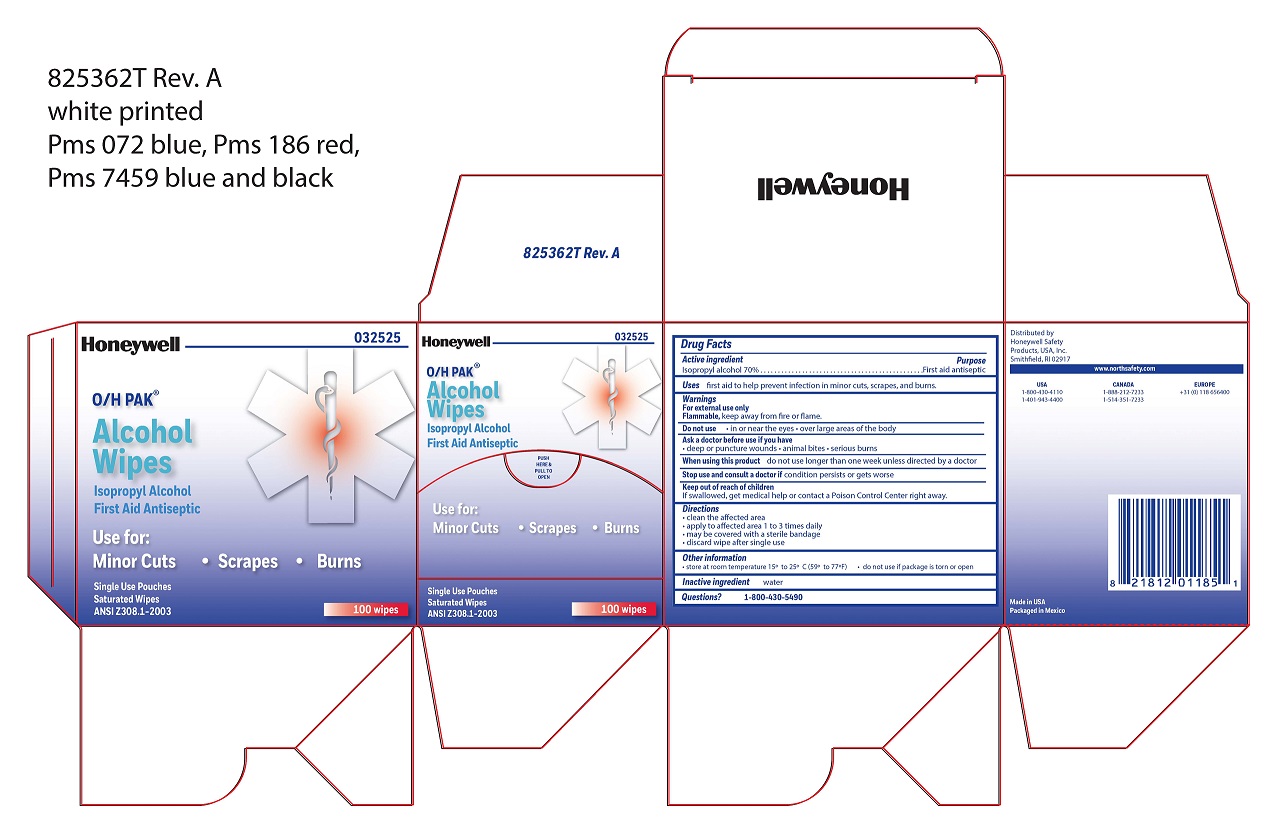
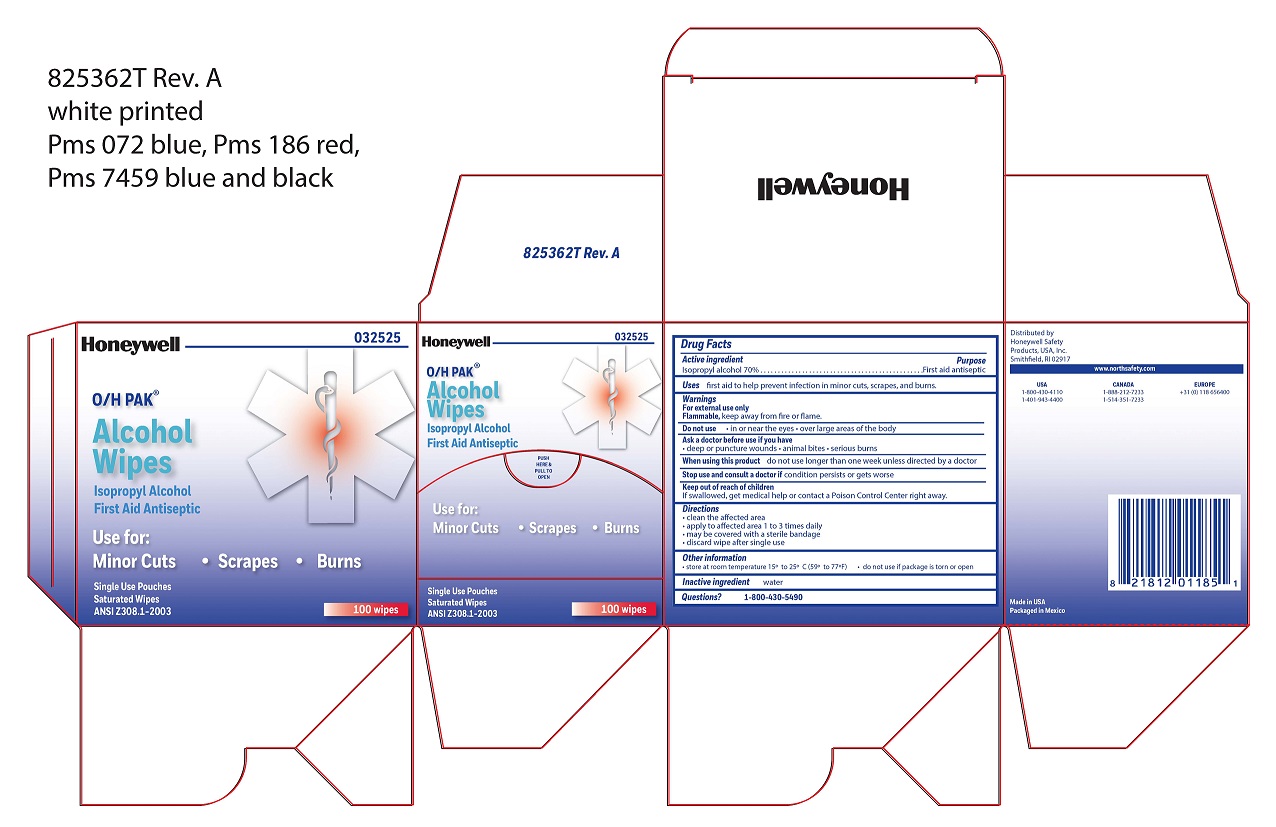
- Alcohol Wipe Principal Display Panel
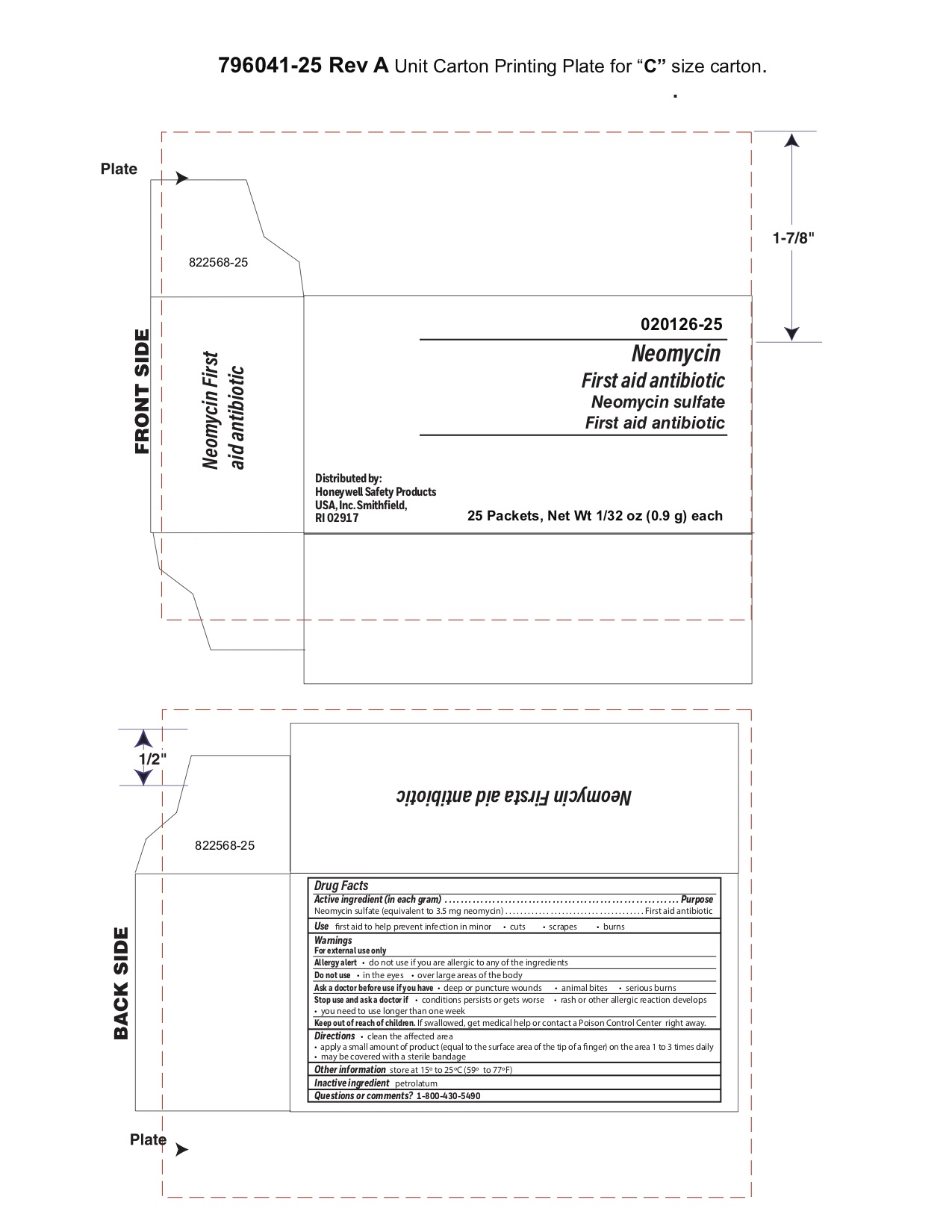
- Neomycin Principal Display Panel
- Antiseptic Spray Principal Display Panel
- Burn Spray Principal Display Panel
- Hand sanitizer Principal Display Panel
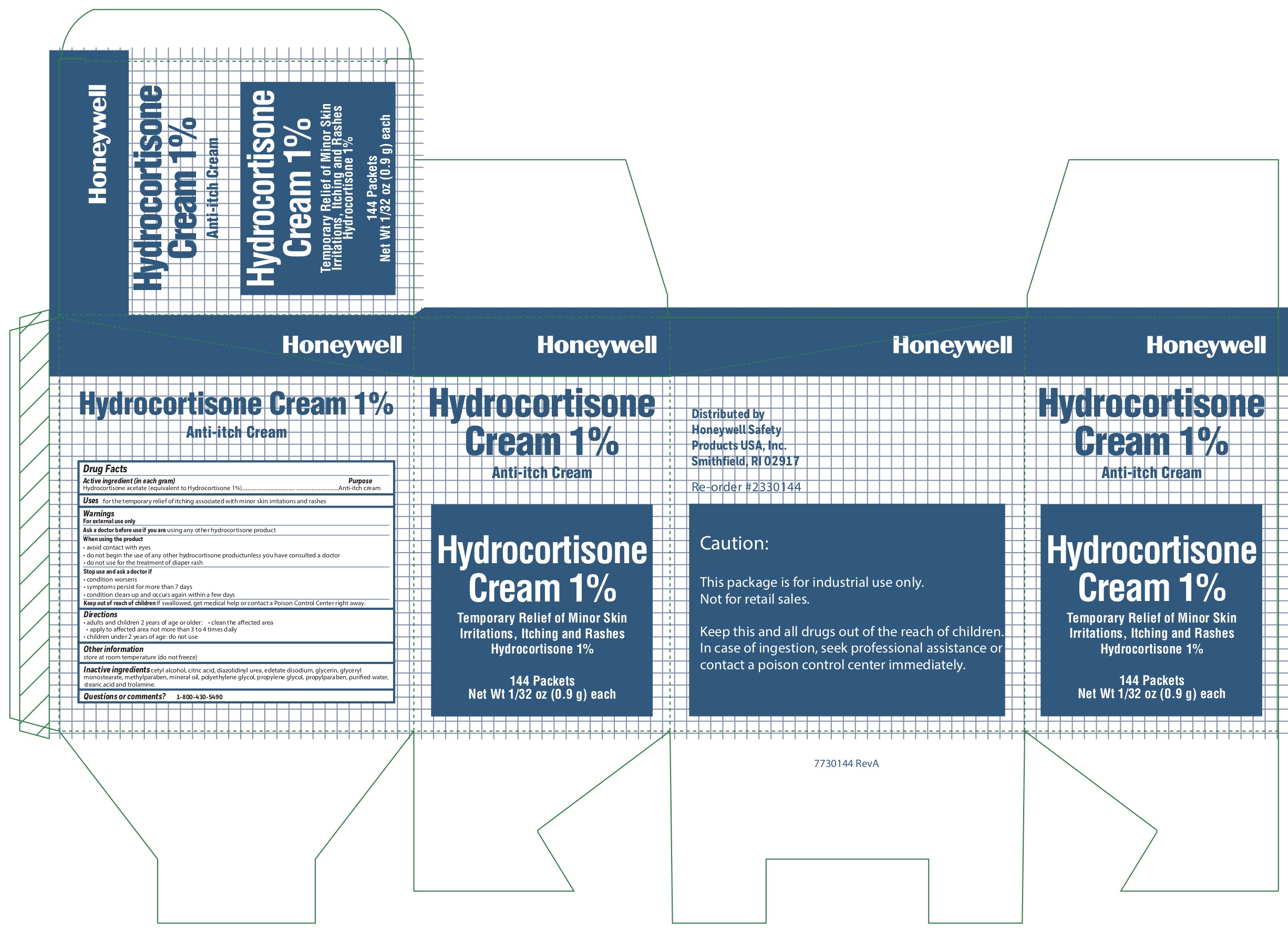
- Hydrocortisone Principal Display Panel
- 4360 Kit Label FRKSOFTPAK-CLSB
-
4360 Kit Contnets
FRKSOFTPAK-CLSB
1 1 X 3 WOVEN 100/BOX
3 NEOMYCIN ANTIBIOTIC 10 PER
2 EYE DRESS PKT W/4 ADH STRIPS
1 TOURNIQUET, 1 PER
1 WIRE SPLINT 1 PER
1 RESCUE BLANKET 1 PER
1 GAUZE COMP, 18" X 36", 2 PER
1 BANDAGE COMP, 2" OFFSET, 4 PER
1 ALCOHOL PREP PADS 10P
1 HYDROCORTISON,1.O%,1/32 OZ,10P
1 O/H PAK,ADH BDG 2"X4", X-LG,10 PER
1 O/H PUMP ANTISEPTIC 2 OZ ID F
1 O/H PUMP BURN RELIEF 2 OZ ID G
1 FIRST AID GUIDE ASHI
1 TAPE ADHESIVE 1"X 5 YD PLSTC
10 HAND SANITIZER 0.9G WJ BULK
2 GAUZE CLEAN-WRAP BDGE N/S 2"
1 GAUZE CLEAN-WRAP BDGE N/S 4"
4 BLOODSTOPPER
1 GZE PADS STERILE 3"X 3" 10'S
1 ELASTIC BANDAGE 3" X 4.5YD
1 CPR FILTERSHIELD 77-100
1 4OZ BFS EYEWASH TRILINGUAL BOTTLE
1 SCISSOR BDGE 4" RED PLS HDL
1 KIT TWEEZER 3 1/2" SLANTED
LBL STOCK 6-3/8"X4"
LBL STOCK 4"X2-7/8"
1 LBL STOCK 3"x1-7/8"
4 PR LRG NITRILE GLVES
2 WATER-JEL BURN DRESSING 4 X 4
1 KIT BAG SOFT PACK LARGE
1 LBL CONTENTS ANSI 2015 CL B
2 TRI BNDG NON WOVEN 40"X40"X56"
2 COLD PACK UNIT 4"X6" BULK
4 WOVEN FINGERTIP BANDAGE 2"
6 WOVEN KNUCKLE BANDAGE
-
INGREDIENTS AND APPEARANCE
4360 FIRST AID KIT
4360 first aid kit kitProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:0498-4360 Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:0498-4360-01 1 in 1 KIT; Type 0: Not a Combination Product 10/18/2018 Quantity of Parts Part # Package Quantity Total Product Quantity Part 1 1 BOTTLE 118 mL Part 2 10 POUCH 4 mL Part 3 10 PACKET 9 g Part 4 1 BOTTLE, SPRAY 59 mL Part 5 1 BOTTLE, SPRAY 59 mL Part 6 30 PACKET 27 g Part 7 10 PACKAGE 9 mL Part 1 of 7 EYESALINE EMERGENCY EYEWASH
purified water liquidProduct Information Item Code (Source) NDC:0498-0100 Route of Administration OPHTHALMIC Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength WATER (UNII: 059QF0KO0R) (WATER - UNII:059QF0KO0R) WATER 98.6 mL in 100 mL Inactive Ingredients Ingredient Name Strength SODIUM CHLORIDE (UNII: 451W47IQ8X) SODIUM PHOSPHATE, DIBASIC (UNII: GR686LBA74) SODIUM PHOSPHATE, MONOBASIC, MONOHYDRATE (UNII: 593YOG76RN) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:0498-0100-02 118 mL in 1 BOTTLE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M018 12/18/2018 Part 2 of 7 ALCOHOL WIPE
isopropyl alcohol swabProduct Information Item Code (Source) NDC:0498-0143 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ISOPROPYL ALCOHOL (UNII: ND2M416302) (ISOPROPYL ALCOHOL - UNII:ND2M416302) ISOPROPYL ALCOHOL 0.7 mL in 1 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:0498-0143-04 0.4 mL in 1 POUCH; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved drug other 09/18/2018 Part 3 of 7 HYDROCORTISONE
anti-itch creamProduct Information Item Code (Source) NDC:0498-0801 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength HYDROCORTISONE ACETATE (UNII: 3X7931PO74) (HYDROCORTISONE - UNII:WI4X0X7BPJ) HYDROCORTISONE ACETATE 1 g in 100 g Inactive Ingredients Ingredient Name Strength METHYLPARABEN (UNII: A2I8C7HI9T) WATER (UNII: 059QF0KO0R) PROPYLPARABEN (UNII: Z8IX2SC1OH) CITRIC ACID MONOHYDRATE (UNII: 2968PHW8QP) LIGHT MINERAL OIL (UNII: N6K5787QVP) GLYCERYL MONOSTEARATE (UNII: 230OU9XXE4) CETYL ALCOHOL (UNII: 936JST6JCN) TROLAMINE (UNII: 9O3K93S3TK) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) POLYETHYLENE GLYCOL, UNSPECIFIED (UNII: 3WJQ0SDW1A) EDETATE DISODIUM (UNII: 7FLD91C86K) STEARIC ACID (UNII: 4ELV7Z65AP) DIAZOLIDINYL UREA (UNII: H5RIZ3MPW4) GLYCERIN (UNII: PDC6A3C0OX) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:0498-0801-35 0.9 g in 1 PACKET; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved drug other 10/15/2019 Part 4 of 7 BURN RELIEF
lidocaine hydrochloride sprayProduct Information Item Code (Source) NDC:0498-0221 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength LIDOCAINE HYDROCHLORIDE (UNII: V13007Z41A) (LIDOCAINE - UNII:98PI200987) LIDOCAINE HYDROCHLORIDE ANHYDROUS 24.64 mg in 1 mL Inactive Ingredients Ingredient Name Strength EDETATE DISODIUM (UNII: 7FLD91C86K) GLYCERIN (UNII: PDC6A3C0OX) TROLAMINE (UNII: 9O3K93S3TK) PROPYLPARABEN (UNII: Z8IX2SC1OH) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) WATER (UNII: 059QF0KO0R) OCTOXYNOL-9 (UNII: 7JPC6Y25QS) HYPROMELLOSES (UNII: 3NXW29V3WO) TEA TREE OIL (UNII: VIF565UC2G) METHYLPARABEN (UNII: A2I8C7HI9T) DIAZOLIDINYL UREA (UNII: H5RIZ3MPW4) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:0498-0221-59 59 mL in 1 BOTTLE, SPRAY; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved drug other 09/18/2018 Part 5 of 7 ANTISEPTIC
benzalkonium chloride sprayProduct Information Item Code (Source) NDC:0498-0402 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength BENZALKONIUM CHLORIDE (UNII: F5UM2KM3W7) (BENZALKONIUM - UNII:7N6JUD5X6Y) BENZALKONIUM CHLORIDE 0.13 g in 100 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) OCTOXYNOL 9 (UNII: 7JPC6Y25QS) GLYCERIN (UNII: PDC6A3C0OX) DIAZOLIDINYL UREA (UNII: H5RIZ3MPW4) DIPROPYLENE GLYCOL (UNII: E107L85C40) EDETATE DISODIUM (UNII: 7FLD91C86K) TROLAMINE (UNII: 9O3K93S3TK) METHYLPARABEN (UNII: A2I8C7HI9T) PROPYLPARABEN (UNII: Z8IX2SC1OH) HYPROMELLOSE, UNSPECIFIED (UNII: 3NXW29V3WO) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:0498-0402-59 59 mL in 1 BOTTLE, SPRAY; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved drug other 09/18/2018 Part 6 of 7 NEOMYCIN
antibiotic ointmentProduct Information Item Code (Source) NDC:0498-0730 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength NEOMYCIN SULFATE (UNII: 057Y626693) (NEOMYCIN - UNII:I16QD7X297) NEOMYCIN SULFATE 3.5 mg in 1 g Inactive Ingredients Ingredient Name Strength PETROLATUM (UNII: 4T6H12BN9U) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:0498-0730-01 0.9 g in 1 PACKET; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved drug other 03/31/2010 Part 7 of 7 INSTANT HAND SANITIZER
alcohol liquidProduct Information Item Code (Source) NDC:59898-420 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ALCOHOL (UNII: 3K9958V90M) (ALCOHOL - UNII:3K9958V90M) ALCOHOL 62 mL in 100 mL Inactive Ingredients Ingredient Name Strength ALOE VERA LEAF (UNII: ZY81Z83H0X) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) .ALPHA.-TOCOPHEROL ACETATE, DL- (UNII: WR1WPI7EW8) CARBOMER COPOLYMER TYPE A (UNII: 71DD5V995L) WATER (UNII: 059QF0KO0R) TRIISOPROPANOLAMINE (UNII: W9EN9DLM98) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:59898-420-36 0.9 mL in 1 PACKAGE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved drug other 04/15/2010 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved drug other 10/18/2018 Labeler - Honeywell Safety Products USA, INC (118768815)