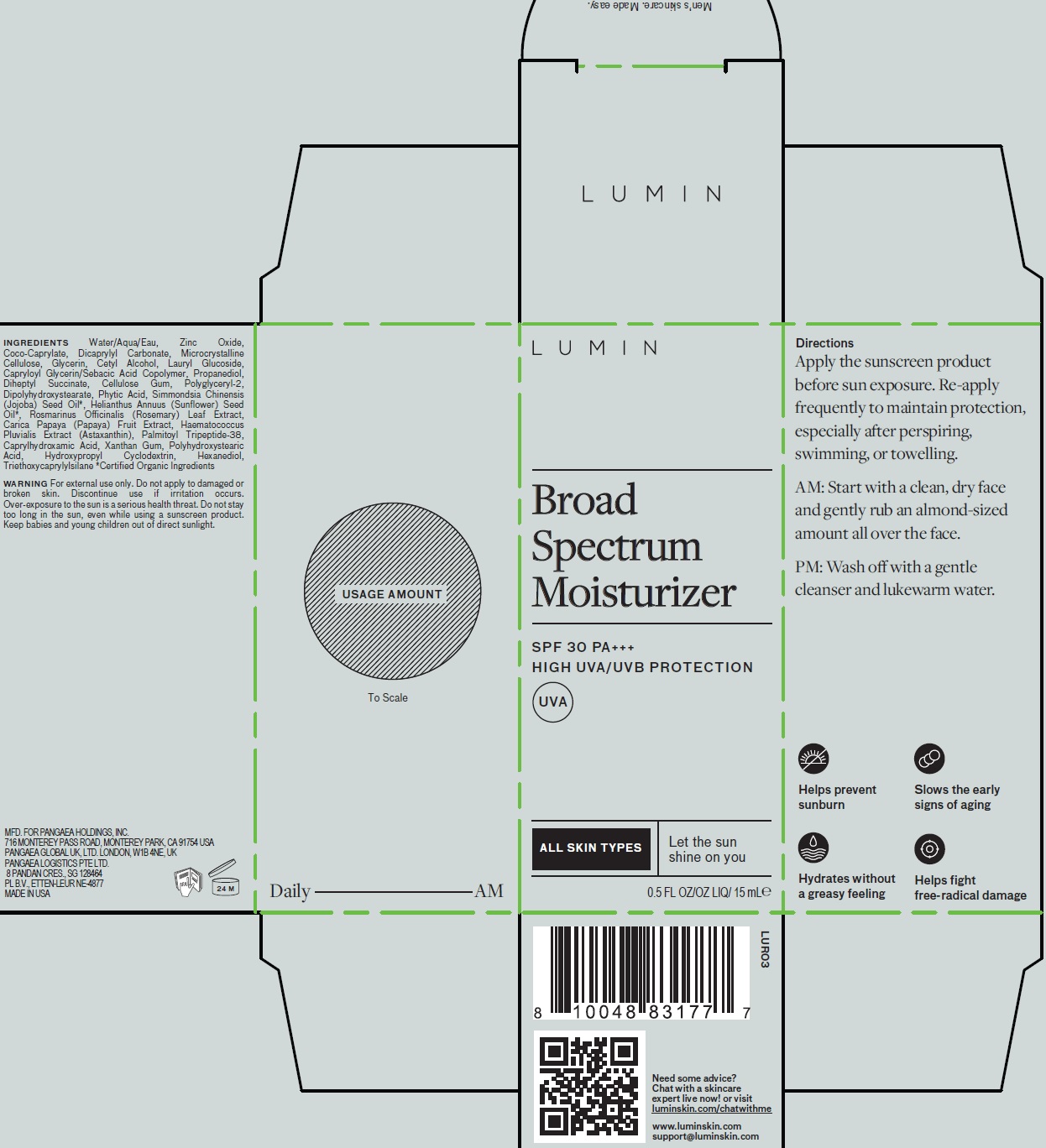
Label: LUMIN BROAD SPECTRUM MOISTURIZER WITH SPF 30 PA HIGH UVA/UVB PROTECTION- zinc oxide cream
- NDC Code(s): 81234-809-00, 81234-809-02
- Packager: Pangaea Holdings Inc.
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: Export only
Drug Label Information
Updated January 19, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
INGREDIENTS
Water/Aqua/Eau, Coco-Caprylate, Dicaprylyl Carbonate, Microcrystalline Cellulose, Glycerin, Cetyl Alcohol, Lauryl Glucoside, Capryloyl Glycerin/Sebacic Acid Copolymer, Propanediol, Diheptyl Succinate, Cellulose Gum, Polyglyceryl-2 Dipolyhydroxystearate, Phytic Acid, Simmondsia Chinensis (Jojoba) Seed Oil*, Helianthus Annuus (Sunflower) Seed Oil*, Rosmarinus Officinalis (Rosemary) Leaf Extract, Carica Papaya (Papaya) Fruit Extract, Haematococcus Pluvialis Extract (Astaxanthin), Palmitoyl Tripeptide-38, Caprylhydroxamic Acid, Xanthan Gum, Polyhydroxystearic Acid, Hydroxypropyl Cyclodextrin, Hexanediol, Triethoxycaprylylsilane
*Certified Organic Ingredients - Active Ingredients
- Warnings
- Directions
- Package Labeling:81234-809-00
- Package Labeling:81234-809-02
-
INGREDIENTS AND APPEARANCE
LUMIN BROAD SPECTRUM MOISTURIZER WITH SPF 30 PA HIGH UVA/UVB PROTECTION
zinc oxide creamProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:81234-809 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ZINC OXIDE (UNII: SOI2LOH54Z) (ZINC CATION - UNII:13S1S8SF37) ZINC CATION 200 mg in 1 mL Inactive Ingredients Ingredient Name Strength HYDROXYPROPYL BETADEX (UNII: 1I96OHX6EK) POLYHYDROXYSTEARIC ACID (2300 MW) (UNII: YXH47AOU0F) WATER (UNII: 059QF0KO0R) COCO-CAPRYLATE (UNII: 4828G836N6) DICAPRYLYL CARBONATE (UNII: 609A3V1SUA) MICROCRYSTALLINE CELLULOSE (UNII: OP1R32D61U) GLYCERIN (UNII: PDC6A3C0OX) CETYL ALCOHOL (UNII: 936JST6JCN) LAURYL GLUCOSIDE (UNII: 76LN7P7UCU) CAPRYLOYL GLYCERIN/SEBACIC ACID COPOLYMER (2000 MPA.S) (UNII: N7YC58165T) PROPANEDIOL (UNII: 5965N8W85T) DIHEPTYL SUCCINATE (UNII: 057N7SS26Y) CARBOXYMETHYLCELLULOSE SODIUM, UNSPECIFIED (UNII: K679OBS311) POLYGLYCERYL-2 DIPOLYHYDROXYSTEARATE (UNII: 9229XJ4V12) FYTIC ACID (UNII: 7IGF0S7R8I) JOJOBA OIL (UNII: 724GKU717M) SUNFLOWER OIL (UNII: 3W1JG795YI) ROSEMARY (UNII: IJ67X351P9) PAPAYA (UNII: KU94FIY6JB) HAEMATOCOCCUS PLUVIALIS (UNII: 31T0FF0472) PALMITOYL LYSYLDIOXYMETHIONYLLYSINE (UNII: T7A529FB8O) CAPRYLHYDROXAMIC ACID (UNII: UPY805K99W) XANTHAN GUM (UNII: TTV12P4NEE) HEXANEDIOL (UNII: ZIA319275I) TRIETHOXYCAPRYLYLSILANE (UNII: LDC331P08E) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:81234-809-00 1 in 1 BOX 01/01/2022 01/05/2025 1 15 mL in 1 BOTTLE, PUMP; Type 0: Not a Combination Product 2 NDC:81234-809-02 1 in 1 BOX 01/01/2022 01/05/2025 2 40 mL in 1 BOTTLE, PUMP; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date Export only 01/01/2022 01/05/2025 Labeler - Pangaea Holdings Inc. (081181313)