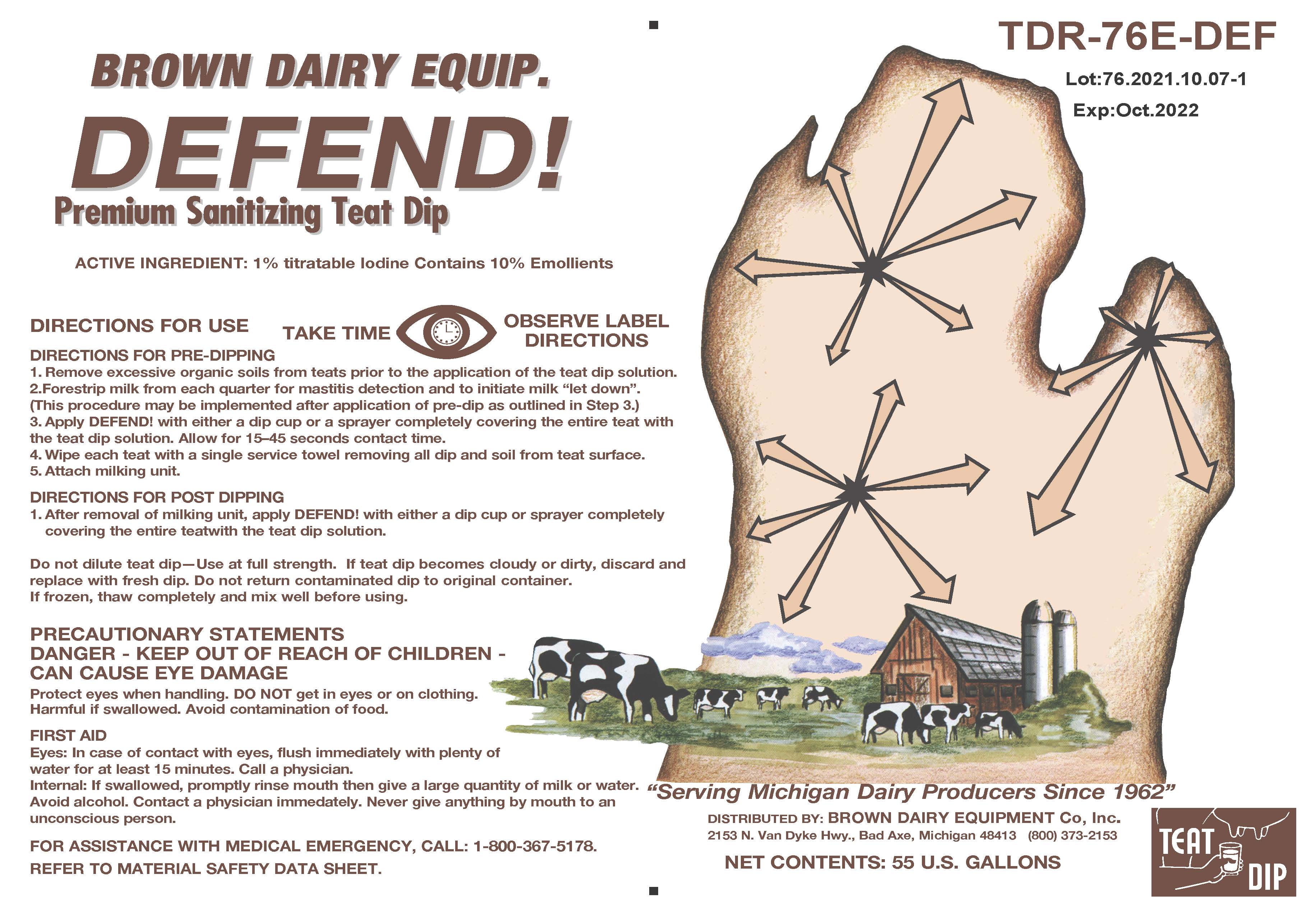
Label: DEFEND- iodine solution
- NDC Code(s): 62897-760-55, 62897-760-75
- Packager: Brown Dairy Equipment
- Category: OTC ANIMAL DRUG LABEL
- DEA Schedule: None
- Marketing Status: unapproved drug other
DISCLAIMER: This drug has not been found by FDA to be safe and effective, and this labeling has not been approved by FDA. For further information about unapproved drugs, click here.
Drug Label Information
Updated November 18, 2021
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- ACTIVE INGREDIENT
- PURPOSE
-
INDICATIONS & USAGE
DIRECTIONS FOR USE
DIRECTIONS FOR PRE-DIPPING
1. Remove excessive organic soils from teats prior to the application of the teat dip solution.
2. Forestrip milk from each quarter for mastitis detection and to initiate milk "let down". (This procedure may be implemented after application of pre-dip as outlined in Step 3.)
3. Apply DEFEND! with either a dip cup or a sprayer completely covering the entire teat with the teat dip solution. Allow for 15-45 seconds contact time.
4. Wipe each teat with a single service towel removing all dip and soil from teat surface.
5. Attach milking unit.
DIRECTIONS FOR POST DIPPING
1. After removal of milking unit, apply DEFEND! with either a dip cup or sprayer competely covering the entire teat with the teat dip solution.
- WARNINGS
-
PRECAUTIONS
PRECAUTIONARY STATEMENTS
CAN CAUSE EYE DAMAGE
Protect eyes when handling. Do NOT get in eyes or on clothing.
Harmful if swallowed. Avoid contamination of food.
FIRST AID
Eyes: In case of contact with eyes, flush immediately with plenty of water for at least 15 minutes. Call a physician.
Internal: If swallowed, promptly rinse mouth then give a large quantity of milk or water. Avoid Alcohol. Contact a physician immediately. Never give anything my mouth to an unconscious person.
- KEEP OUT OF REACH OF CHILDREN
- SPL UNCLASSIFIED SECTION
- SPL UNCLASSIFIED SECTION
- SPL UNCLASSIFIED SECTION
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
DEFEND
iodine solutionProduct Information Product Type OTC ANIMAL DRUG Item Code (Source) NDC:62897-760 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength IODINE (UNII: 9679TC07X4) (IODINE - UNII:9679TC07X4) IODINE 10 g in 1 L Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) SORBITOL (UNII: 506T60A25R) C12-15 PARETH-9 (UNII: H3ZIY6WP1R) XANTHAN GUM (UNII: TTV12P4NEE) POVIDONE K30 (UNII: U725QWY32X) SODIUM HYDROXIDE (UNII: 55X04QC32I) PHOSPHORIC ACID (UNII: E4GA8884NN) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:62897-760-55 208.2 L in 1 DRUM 2 NDC:62897-760-75 1041 L in 1 TANK Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved drug other 11/18/2021 Labeler - Brown Dairy Equipment (017030149) Establishment Name Address ID/FEI Business Operations Dairy Dynamics L.L.C. 142010953 api manufacture, manufacture, pack, label