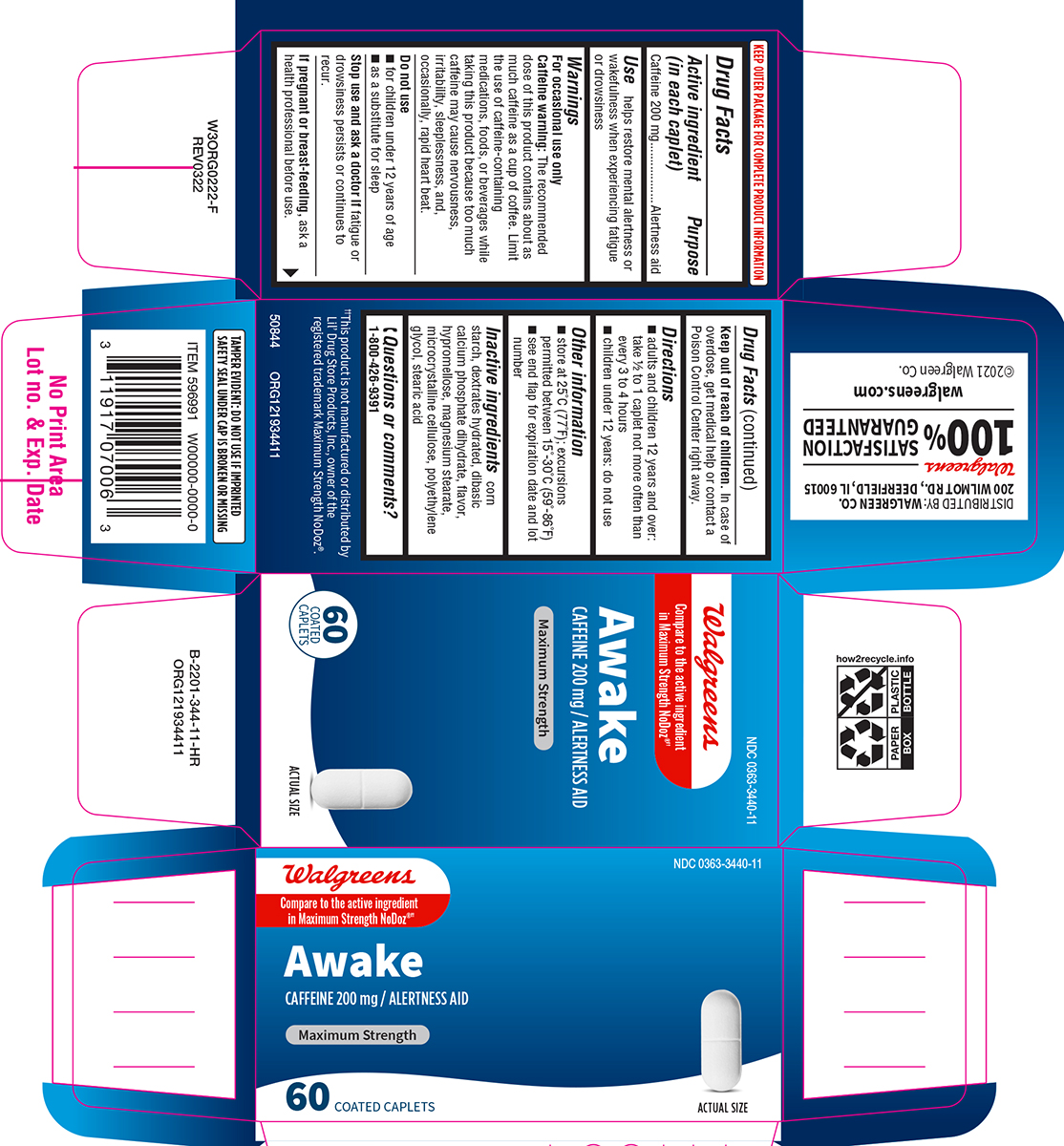
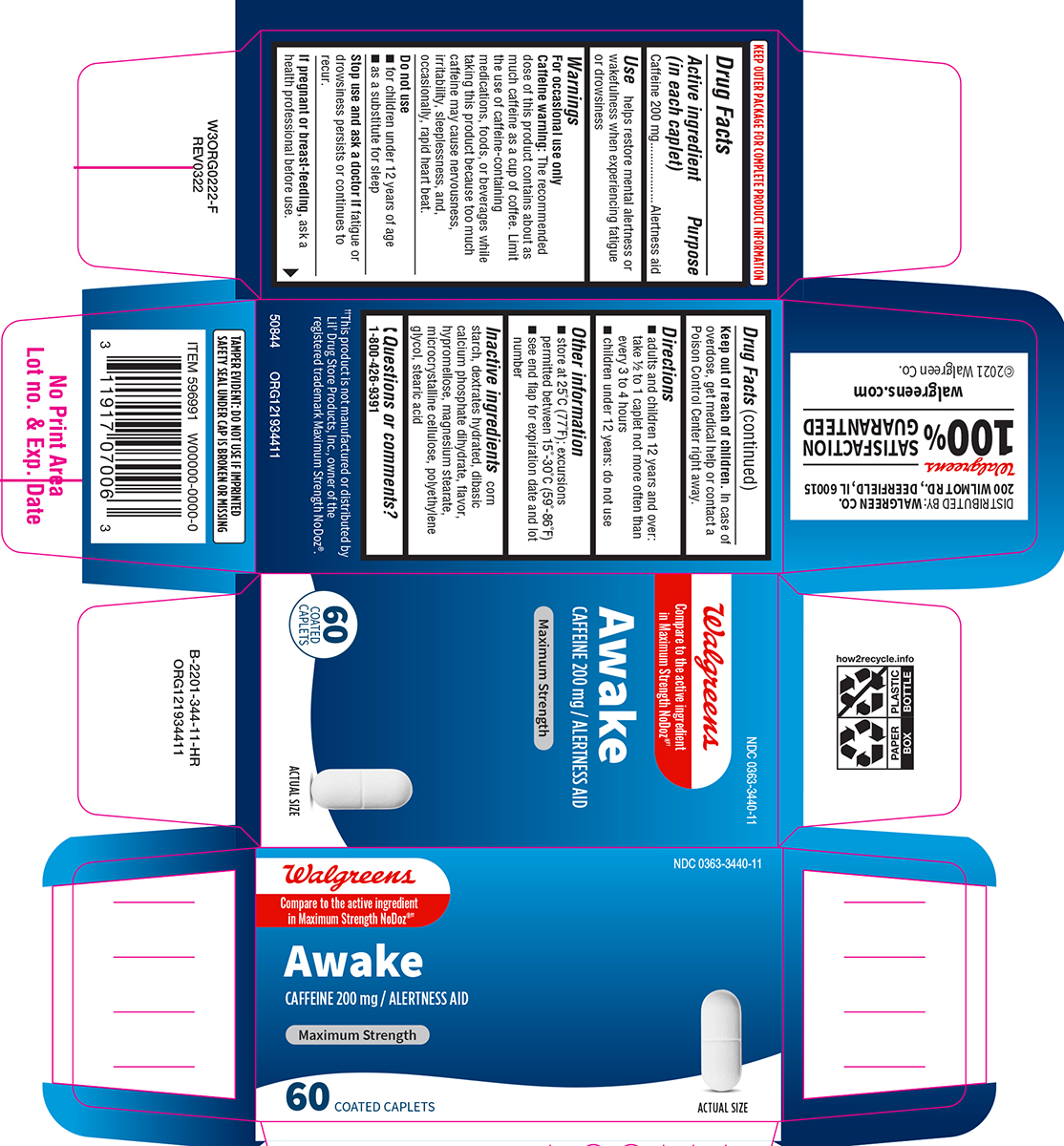
Label: AWAKE MAXIMUM STRENGTH- caffeine tablet, film coated
- NDC Code(s): 0363-3440-11
- Packager: Walgreen Company
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated September 15, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Active ingredient (in each caplet)
- Purpose
- Use
-
Warnings
For occasional use only
Caffeine warning: The recommended dose of this product contains about as much caffeine as a cup of coffee. Limit the use of caffeine-containing medications, foods, or beverages while taking this product because too much caffeine may cause nervousness, irritability, sleeplessness, and, occasionally, rapid heart beat.
- Directions
- Other information
- Inactive ingredients
- Questions or comments?
-
Principal Display Panel
NDC 0363-3440-11
Walgreens
Compare to the active ingredient
in Maximum Strength NoDoz®††Awake
CAFFEINE 200 mg / ALERTNESS AID
MAXIMUM STRENGTH
60 COATED
CAPLETSACTUAL SIZE
TAMPER EVIDENT: DO NOT USE IF IMPRINTED
SAFETY SEAL UNDER CAP IS BROKEN OR MISSING††This product is not manufactured or distributed by
Lil' Drug Store Products. Inc., owner of the
registered trademark Maximum Strength NoDoz®.50844 ORG121934411
DISTRIBUTED BY: WALGREEN CO.
200 WILMOT RD., DEERFIELD, IL 60015Walgreens
100 % SATISFACTION GUARANTEED
walgreens.com ©2021 Walgreen Co.
Walgreens 44-344
-
INGREDIENTS AND APPEARANCE
AWAKE MAXIMUM STRENGTH
caffeine tablet, film coatedProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:0363-3440 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength CAFFEINE (UNII: 3G6A5W338E) (CAFFEINE - UNII:3G6A5W338E) CAFFEINE 200 mg Inactive Ingredients Ingredient Name Strength STARCH, CORN (UNII: O8232NY3SJ) DEXTROSE MONOHYDRATE (UNII: LX22YL083G) DIBASIC CALCIUM PHOSPHATE DIHYDRATE (UNII: O7TSZ97GEP) HYPROMELLOSE, UNSPECIFIED (UNII: 3NXW29V3WO) MAGNESIUM STEARATE (UNII: 70097M6I30) MICROCRYSTALLINE CELLULOSE (UNII: OP1R32D61U) POLYETHYLENE GLYCOL, UNSPECIFIED (UNII: 3WJQ0SDW1A) STEARIC ACID (UNII: 4ELV7Z65AP) Product Characteristics Color white Score 2 pieces Shape OVAL Size 15mm Flavor PEPPERMINT Imprint Code 44;344 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:0363-3440-11 1 in 1 CARTON 04/14/1998 1 60 in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M011 04/14/1998 Labeler - Walgreen Company (008965063) Establishment Name Address ID/FEI Business Operations LNK International, Inc. 038154464 pack(0363-3440) Establishment Name Address ID/FEI Business Operations LNK International, Inc. 832867837 manufacture(0363-3440) Establishment Name Address ID/FEI Business Operations LNK International, Inc. 832867894 manufacture(0363-3440) Establishment Name Address ID/FEI Business Operations LNK International, Inc. 967626305 pack(0363-3440) Establishment Name Address ID/FEI Business Operations LNK International, Inc. 117025878 manufacture(0363-3440)