Label: STERILE WATER- water injection
- NDC Code(s): 0264-7385-50, 0264-7385-60
- Packager: B. Braun Medical Inc.
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: New Drug Application
Drug Label Information
Updated August 6, 2021
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- SPL UNCLASSIFIED SECTION
-
DESCRIPTION
Sterile Water for Injection USP is a clear, colorless, odorless liquid. It is sterile, hypotonic, nonpyrogenic, and contains no bacteriostatic or antimicrobial agents. Sterile Water for Injection USP is a diluent suitable for intravascular injection after first having been made approximately isotonic by the addition of suitable solute.
pH: 5.5 (5.0-7.0)
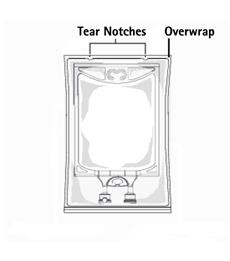
Not made with natural rubber latex, PVC or DEHP.
The plastic container is made from a multilayered film specifically developed for parenteral drugs. It contains no plasticizers and exhibits virtually no leachables. The solution contact layer is a rubberized copolymer of ethylene and propylene. The container is nontoxic and biologically inert. The container-solution unit is a closed system and is not dependent upon entry of external air during use. The container is overwrapped to provide protection from the physical environment and to provide an additional moisture barrier when necessary.
-
CLINICAL PHARMACOLOGY
Sterile Water for Injection USP is used as a diluent for other parenteral drugs. As such, Sterile Water for Injection USP contributes to the water for hydration when provided in parenteral drug and fluid therapy, after the introduction of suitable additives and/or mixture with suitable solutes to approximate isotonicity.
- INDICATIONS AND USAGE
- CONTRAINDICATIONS
-
WARNINGS
This solution is for compounding only, not for direct infusion.
Hypotonic and hemolytic. Do not inject until made approximately isotonic by addition of an appropriate solute, due to the possibility of hemolysis.
The administration of intravenous solutions can cause fluid and/or solute overload resulting in dilution of serum electrolyte concentrations, overhydration, congested states or pulmonary edema. The risk of dilutional states is inversely proportional to the electrolyte concentration.
WARNING: This product contains aluminum that may be toxic. Aluminum may reach toxic levels with prolonged parenteral administration if kidney function is impaired. Premature neonates are particularly at risk because their kidneys are immature, and they require large amounts of calcium and phosphate solutions, which contain aluminum.
Research indicates that patients with impaired kidney function, including premature neonates, who receive parenteral levels of aluminum at greater than 4 to 5 mcg/kg/day accumulate aluminum at levels associated with central nervous system and bone toxicity. Tissue loading may occur at even lower rates of administration.
-
PRECAUTIONS
General
To minimize the risk of possible incompatibilities arising from the mixing of additives that may be prescribed, the final infusate should be inspected for cloudiness or precipitation immediately after mixing, prior to administration and periodically during administration.
Do not use plastic container in series connection.
If administration of Sterile Water for Injection USP after admixture or dilution is controlled by a pumping device, care must be taken to discontinue pumping action before the container runs dry or air embolism may result. If administration is not controlled by a pumping device, refrain from applying excessive pressure (>300mmHg) causing distortion to the container such as wringing or twisting. Such handling could result in breakage of the container.
This solution is intended for intravenous administration after admixture or dilution using sterile equipment. It is recommended that intravenous administration apparatus be replaced at least once every 24 hours.
Use only if solution is clear and container and seals are intact.
The drug product contains no more than 25 mcg/L of aluminum.
Carcinogenesis, Mutagenesis, Impairment of Fertility
Refer to the package insert of the solute used.
Pediatric Use
Refer to the package insert of the solute used. See WARNINGS section regarding aluminum.
Geriatric Use
Refer to the package insert of the solute used. See WARNINGS section regarding aluminum.
-
ADVERSE REACTIONS
Refer to the package insert of the solute used.
Reactions which may occur because of the solution or the technique of administration include febrile response, infection at the site of injection, venous thrombosis or phlebitis extending from the site of injection, extravasation and hypervolemia.
The physician should also be alert to the possibility of adverse reactions to drug additives. Prescribing information for drug additives to be administered in this manner should be consulted.
If an adverse reaction does occur, discontinue the infusion, evaluate the patient, institute appropriate therapeutic countermeasures and save the remainder of the fluid for examination if deemed necessary.
-
OVERDOSAGE
Overdosage (hypotonic expansion) is a function of an increase in fluid intake over fluid output, and occurs when the increase in the volume of body fluids is due to water alone. Overdosage may occur in patients who receive large quantities of electrolyte-free water to replace abnormal excessive fluid losses, in patients whose renal tolerance to water loads is exceeded, or in patients who retain water postoperatively in response to stress.
Manifestations of water intoxication are behavioral changes (confusion, apathy, disorientation and attendant hyponatremia), central nervous system disturbances (weakness, muscle twitching, headaches, nausea, vomiting, convulsions) and weight gain.
Treatment consists of withholding fluids until excessive water is excreted. In severe hyponatremia it may be necessary to cautiously administer hypertonic saline to increase extracellular osmotic pressure and excretion of excess water by the kidneys.
-
DOSAGE AND ADMINISTRATION
This solution is for intravenous use only after admixture or dilution. Do not inject until made approximately isotonic by addition of appropriate solute.
Following suitable admixture of prescribed drugs, the dosage is usually dependent upon the age, weight and clinical condition of the patient as well as laboratory determinations. See directions accompanying drugs.
The dosage and administration of Sterile Water for Injection USP is dependent upon the recommended dosage and administration of the solute used. Fluid administration should be based on calculated maintenance or replacement fluid requirements for each patient.
Some additives may be incompatible. Consult with pharmacist. When performing admixture or dilution, use aseptic techniques. Mix thoroughly. Do not store.
Parenteral drug products should be inspected visually for particulate matter and discoloration prior to administration, whenever solution and container permit.
- HOW SUPPLIED
- SPL UNCLASSIFIED SECTION
-
Directions for use of Plastic Container
Warning: Hypotonic and hemolytic. Do not inject until made approximately isotonic by addition of appropriate solute.
Parenteral drug products should be inspected visually for particulate matter and discoloration prior to administration and final infusate should be inspected for cloudiness or precipitation immediately after mixing, prior to administration, and periodically during administration, whenever solution and container permit. Use of a final filter is recommended during administration of all parenteral solutions where possible.
Sterile Water for Injection USP in the Pharmacy Bulk Package is intended for use in the preparation of sterile, intravenous admixtures. Additives may be incompatible with the fluid withdrawn from this container. Complete information is not available. Those additives known to be incompatible should not be used. Consult with pharmacist, if available.
When compounding admixtures, use aseptic technique. Mix thoroughly.
Do not store any unused portion of Sterile Water for Injection USP.
Preparation
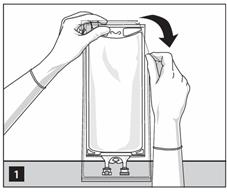
- Inspect overwrap and primary bag.
- Do not use if overwrap has been damaged.
- Do not use unless solution is clear and closure is intact.
1. To open: Tear overwrap starting from the tear notches. (Figure 1)
For Pharmacy Bulk Packages
- The Pharmacy Bulk Package is to be used only in a suitable work area such as a laminar air flow hood (or an equivalent clean air compounding area).
- For compounding only. Do not use for direct infusion.
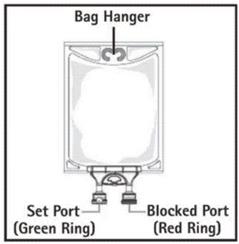
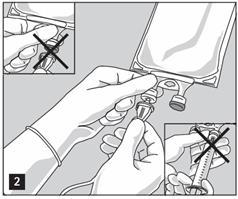
- Do not use/penetrate blocked port (see Figure 2, left upper corner).
- Suspend container.
- Remove aluminum foil of set port at the bottom of container (see Figure 3).
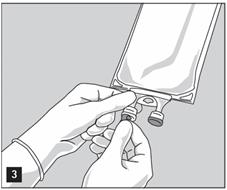
- Attach suitable transfer device or compounding set (Figure 2). Refer to complete directions accompanying device.
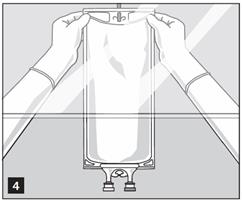
- Hang bag on suitable fixture (Figure 4).
- Once container closure has been penetrated, withdrawal of content should be completed within 4 hours.
Important Admixing Instructions
- The contents are intended for use in a pharmacy admixture program and are restricted to the preparation of admixtures for infusion or, through a sterile transfer device, for the filling of empty sterile syringes.
- Additives may be incompatible with the fluid withdrawn from this container. Consult with pharmacist, if available. When compounding admixtures, use aseptic technique, mix thoroughly and do not store.
- Parenteral drug products should be inspected visually for particulate matter and discoloration prior to administration, whenever solution container permits. (See PRECAUTIONS, General).
-
SPL UNCLASSIFIED SECTION
B. Braun Medical Inc.
Bethlehem, PA 18018-3524 USA
1-800-227-2862
www.bbraun.com
Made in USAY36-002-998 LD-454-2
-
PRINCIPAL DISPLAY PANEL - 2000 mL
STERILE WATER FOR
INJECTION USPWARNING: HYPOTONIC AND HEMOLYTIC.
DO NOT INJECT UNTIL MADE
APPROXIMATELY ISOTONIC BY ADDITION OF
APPROPRIATE SOLUTE.REF S8505
NDC 0264-7385-50
LOT
EXP.2000 mL
Pharmacy Bulk Package
Not For Direct Infusion
No antimicrobial agent or other substance has been added.
Sterile. Pharmacy bulk package container.
WARNINGS: Some additives may be incompatible.
Consult with pharmacist. When introducing additives,
use aseptic techniques. Mix thoroughly. Do not store.CONTAINS NO MORE THAN 25 mcg/L OF ALUMINUM
Recommended Storage: Room temperature (25°C). Avoid
excessive heat. Protect from freezing. Do not remove overwrap
until ready for use. Once container closure has been
penetrated, withdrawal of content should be completed
within 4 hours. See Package Insert.Not made with natural rubber latex, PVC or DEHP.
Rx only

Y38-000-065 LD-455-3
B. Braun Medical Inc.
Bethlehem, PA 18018-3524 USA
1-800-227-2862SET

Sterile Water Injection 2L Container Label
-
PRINCIPAL DISPLAY PANEL - 3000 mL
STERILE WATER
FOR INJECTION USPWARNING: HYPOTONIC AND HEMOLYTIC.
DO NOT INJECT UNTIL MADE
APPROXIMATELY ISOTONIC BY ADDITION OF
APPROPRIATE SOLUTE.REF S8506
NDC 0264-7385-60
LOT
EXP.3000 mL
Pharmacy Bulk Package
Not For Direct InfusionNo antimicrobial agent or other substance has been added.
Sterile. Pharmacy bulk package container.
WARNINGS: Some additives may be incompatible.
Consult with pharmacist. When introducing additives,
use aseptic techniques. Mix thoroughly. Do not store.CONTAINS NO MORE THAN 25 mcg/L OF ALUMINUM
Recommended Storage: Room temperature (25°C).
Avoid excessive heat. Protect from freezing. Do not
remove overwrap until ready for use. Once container
closure has been penetrated, withdrawal of
content should be completed within 4 hours.
See Package Insert.Not made with natural rubber latex, PVC or DEHP.
Rx only

Y38-000-066 LD-456-3
B. Braun Medical Inc.
Bethlehem, PA 18018-3524 USA
1-800-227-2862SET

-
INGREDIENTS AND APPEARANCE
STERILE WATER
water injectionProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:0264-7385 Route of Administration INTRAVENOUS Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength WATER (UNII: 059QF0KO0R) (WATER - UNII:059QF0KO0R) WATER 1 mL in 1 mL Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:0264-7385-50 4 in 1 CASE 09/04/2014 1 2000 mL in 1 CONTAINER; Type 0: Not a Combination Product 2 NDC:0264-7385-60 4 in 1 CASE 09/04/2014 2 3000 mL in 1 CONTAINER; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA019633 09/04/2014 Labeler - B. Braun Medical Inc. (002397347)




