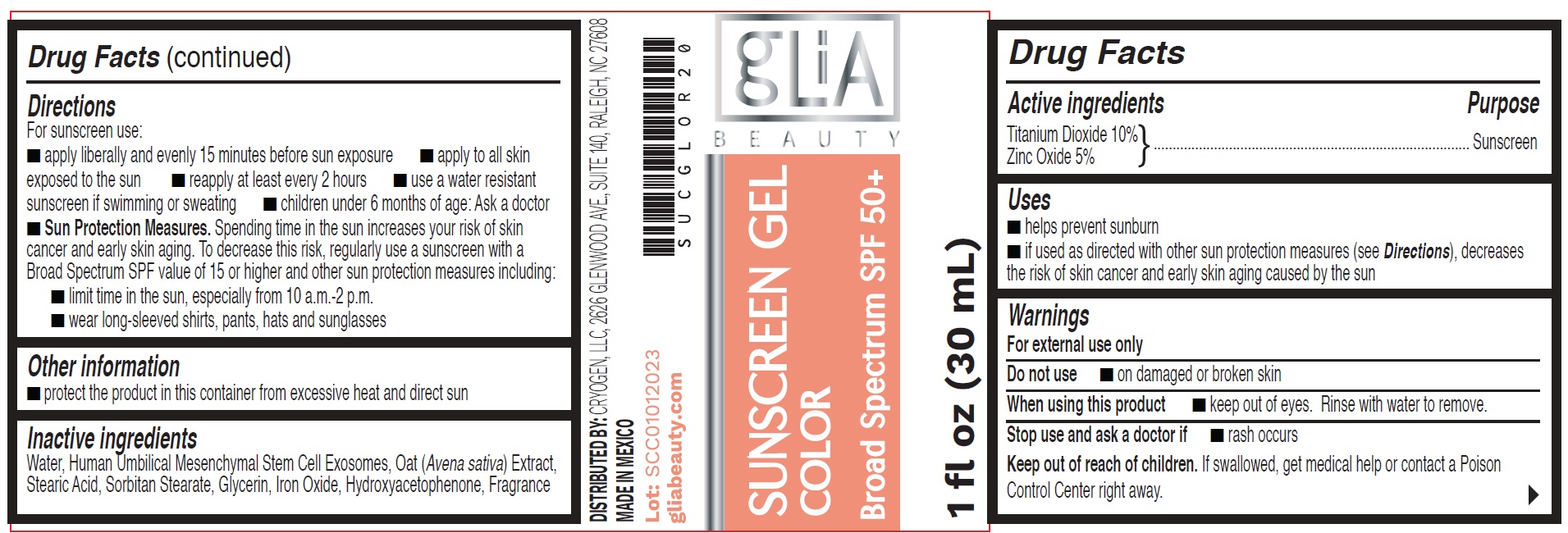
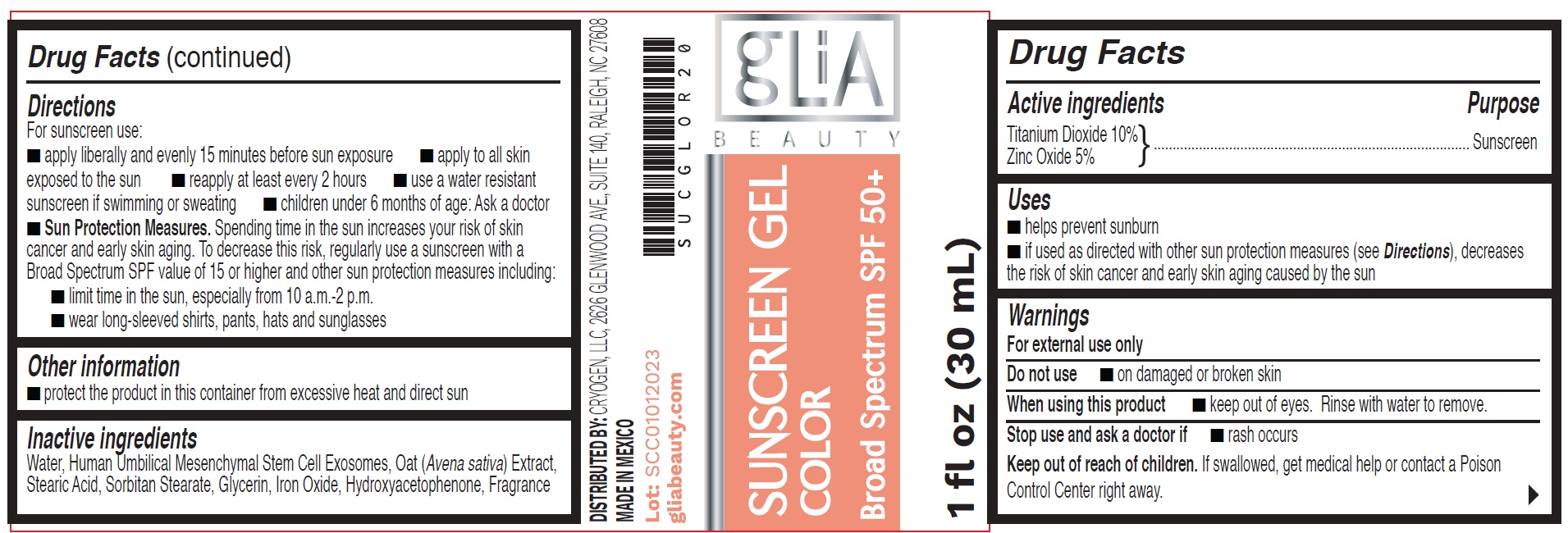
Label: SUNSCREEN COLOR BROAD SPECTRUM SPF 50- titanium dioxide, zinc oxide gel
- NDC Code(s): 83802-001-01
- Packager: CryoGen, LLC
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated November 17, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Drug Facts
- Active ingredients
- Uses
- Warnings
-
Directions
For sunscreen use:
- apply liberally and evenly 15 minutes before sun exposure
- apply to all skin exposed to the sun
- reapply at least every 2 hours
- use a water resistant sunscreen if swimming or sweating
- children under 6 months of age: Ask a doctor
- Sun Protection Measures.Spending time in the sun increases your risk of skin cancer and early skin aging. To decrease this risk, regularly use a sunscreen with a Broad Spectrum SPF value of 15 or higher and other sun protection measures including:
- limit time in the sun, especially from 10 a.m.-2 p.m.
- wear long-sleeved shirts, pants, hats and sunglasses
- Other information
- Inactive ingredients
- Package Labeling:
-
INGREDIENTS AND APPEARANCE
SUNSCREEN COLOR BROAD SPECTRUM SPF 50
titanium dioxide, zinc oxide gelProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:83802-001 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength TITANIUM DIOXIDE (UNII: 15FIX9V2JP) (TITANIUM DIOXIDE - UNII:15FIX9V2JP) TITANIUM DIOXIDE 100 mg in 1 mL ZINC OXIDE (UNII: SOI2LOH54Z) (ZINC CATION - UNII:13S1S8SF37) ZINC CATION 50 mg in 1 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) OAT (UNII: Z6J799EAJK) STEARIC ACID (UNII: 4ELV7Z65AP) SORBITAN MONOSTEARATE (UNII: NVZ4I0H58X) GLYCERIN (UNII: PDC6A3C0OX) FERRIC OXIDE RED (UNII: 1K09F3G675) HYDROXYACETOPHENONE (UNII: G1L3HT4CMH) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:83802-001-01 30 mL in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product 12/01/2023 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M020 12/01/2023 Labeler - CryoGen, LLC (127842726)