Label: 4331 FIRST AID KIT kit
-
NDC Code(s):
0498-0100-02,
0498-0121-00,
0498-0143-04,
0498-4331-01, view more59898-200-12
- Packager: Honeywell Safety Products USA, INC
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: unapproved drug other
DISCLAIMER: This drug has not been found by FDA to be safe and effective, and this labeling has not been approved by FDA. For further information about unapproved drugs, click here.
Drug Label Information
Updated January 22, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Eyesaline Active ingredient
- Eyesaline Purpose
- Eyesaline Uses
-
Eyesaline
Warnings
For external use only-
Obtain immediate medical treatment for all open wounds in or near eyes.
To avoid contamination, do not touch tip of container to any surface.
Do not reuse. Once opened, discard.
Do not use
- if solution changes color or becomes cloudy
- if you have open wounds in or near the eyes, get medical help right away.
- Eyesaline Directions
- Eyesaline Inactive ingredients
- Eyesaline Questions
- Burn Jel Active ingredient
- Burn Jel Purpose
- Burn Jel Uses
- Burn Jel Warnings
- Burn Jel Directions
- Burn Jel Other information
- Burn Jel Inactive ingredients
- Burn Jel Questions
- Povidone Iodine Swab Active ingredient
- Povidone Iodine Swab Purpose
- Povidone Iodine Swab Uses
- Povidone Iodine Swab Warnings
- Povidone Iodine Swab Directions
- Povidone Iodine Swab Other information
- Povidone Iodine Swab Inactive ingredients
- Povidone Iodine Swab Questions and comments
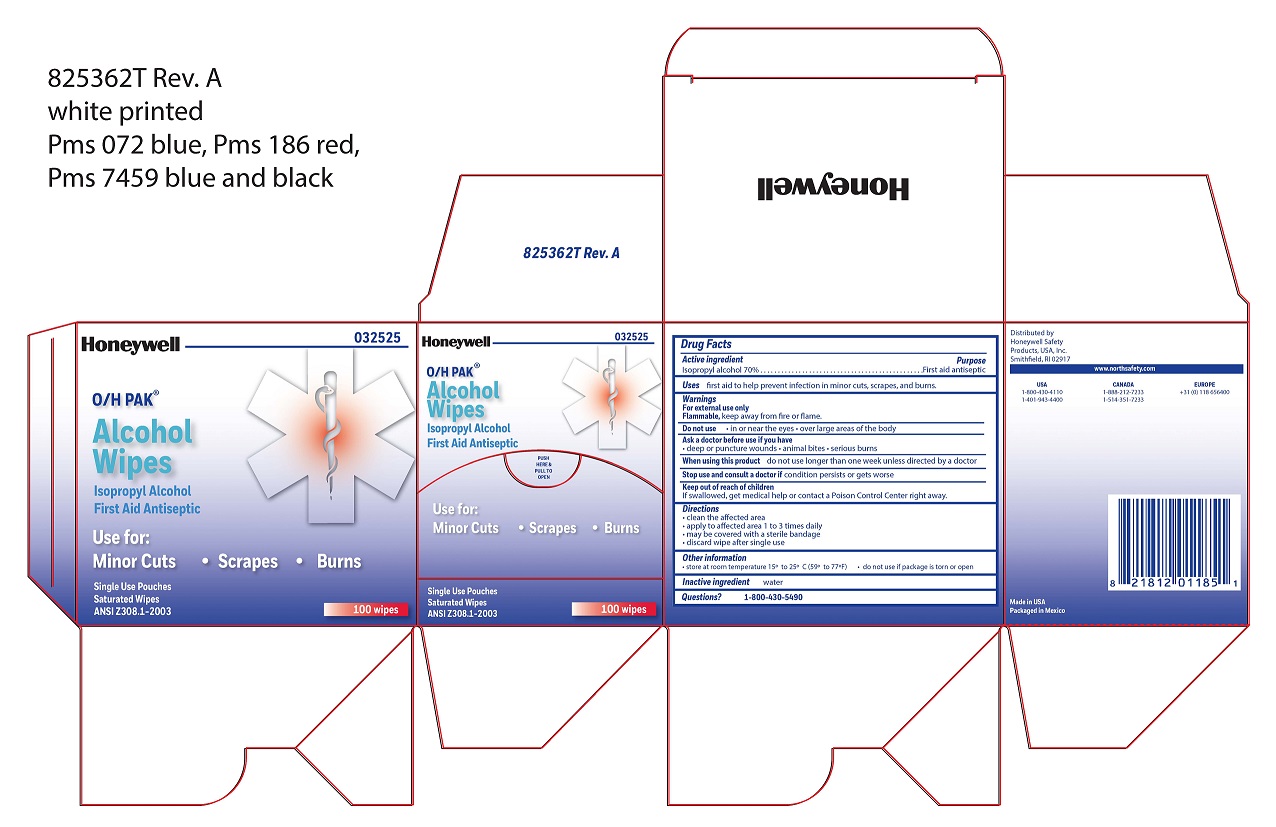
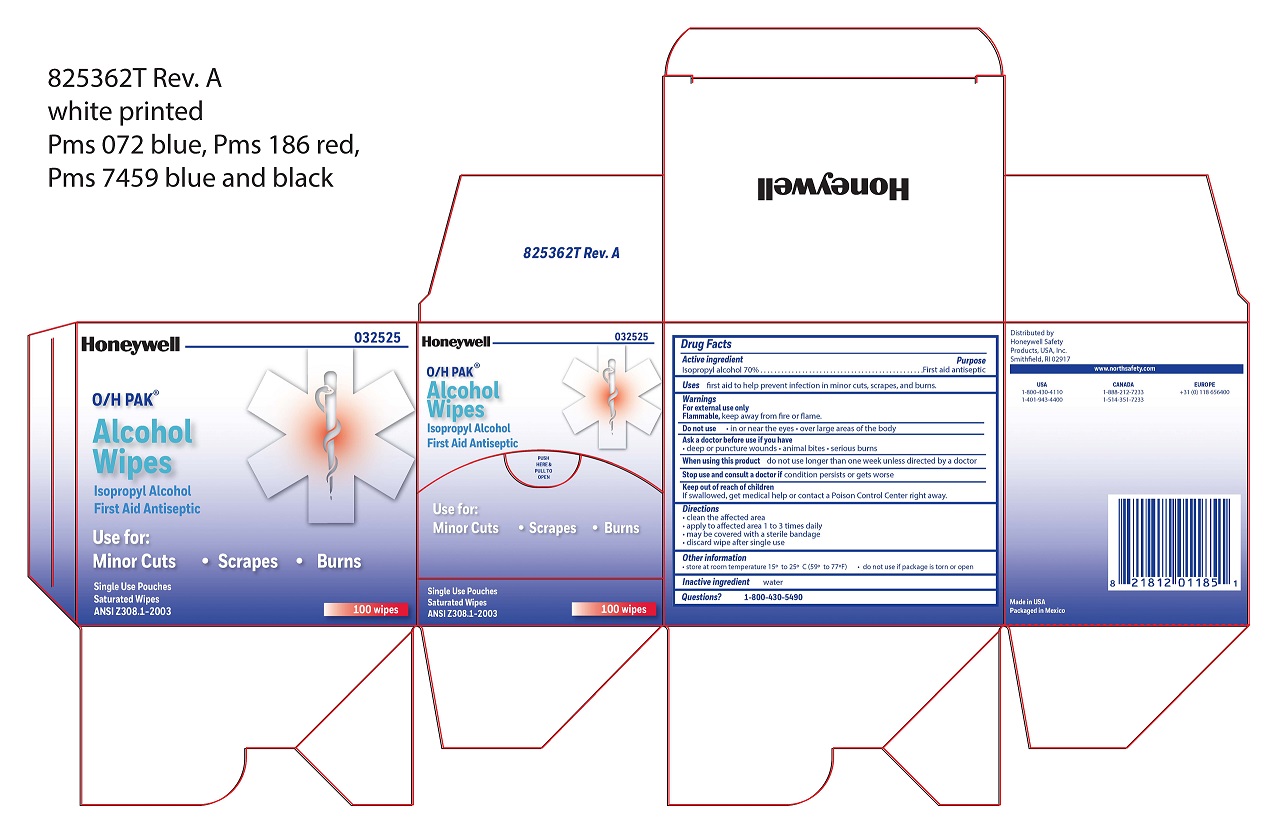
- Alcohol Wipe Active ingredient
- Alcohol Wipe Purpose
- Alcohol Wipe Uses
- Alcohol Wipe Warnings
- Alcohol Wipe Directions
- Alcohol Wipe Other information
- Alcohol Wipe Inactive ingredient
- Alcohol Wipe Questions
-
4331
Z019811 Kit Contents
12 PVP IODINE WIPES 10 PER
1 ADHESIVE TAPE W/P 1" X 10YDS
3 ADH BDG, CLOTH, 1"X3", 16 PER
1 POR. CLOTH TAPE 3X10YD
1 FIRST AID GUIDE ASHI
1 EMERGENCY SURVIVAL BLANKET
2 GAUZE CLEAN-WRAP BDGE N/S 4"
1 GAUZE CLEAN-WRAP BDG N/S 6"
2 BLOODSTOPPER
3 ABD COMBINE PAD 5" X 9"
2 ABD PADS 8"X10" STERILE
1 MULTI-TRAUMA DRESSING 12"X30"
1 ELASTIC BANDAGE 3" X 4.5YD
2 CPR FILTERSHIELD 77-100
1 WATER JEL BURN JEL 4OZ/BTL
2 4OZ BFS EYEWASH TRILINGUAL BOTTLE
1 SCISSOR UTILITY SHEARS 7-1/4"
1 LBL STOCK 4"X2-7/8"
1 LBL CONTS 6 3/4"X3 1/2" ID B
1 LBL STOCK PLAIN 3.2"x7/8"
1 SOFT PACK, CLOTH BAG- LARGE
4 PR LRG NITRILE GLVES
20 WIPE ALCOHOL PREP IPA 70% (DUKAL)
1 BAG BIOHAZARD 24 X 24 RED
4 TRI BNDG NON WOVEN 40"X40"X56"
3 COLD PACK UNIT 4"X6" BULK
4 EYE PADS STD OVAL STERILE
3 GAUZE PADS 3"X3" 12PLY
10 GAUZE PADS 4"X4" 12PLY
4 ZIP LOCK BAG 8 X 10" 2 MIL
- Eyesaline Principal Display Panel
- Burn Jel Principal Display Panel
- Povidone Iodine Swab Principal Display Panel
- Alcohol Wipe Principal Display Panel
- 4331 Kit Label Z019811
-
INGREDIENTS AND APPEARANCE
4331 FIRST AID KIT
4331 first aid kit kitProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:0498-4331 Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:0498-4331-01 1 in 1 KIT; Type 0: Not a Combination Product 10/18/2018 Quantity of Parts Part # Package Quantity Total Product Quantity Part 1 2 BOTTLE 236 mL Part 2 20 POUCH 8 mL Part 3 120 POUCH 36 mL Part 4 1 BOTTLE, PLASTIC 118 mL Part 1 of 4 EYESALINE EMERGENCY EYEWASH
purified water liquidProduct Information Item Code (Source) NDC:0498-0100 Route of Administration OPHTHALMIC Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength WATER (UNII: 059QF0KO0R) (WATER - UNII:059QF0KO0R) WATER 98.6 mL in 100 mL Inactive Ingredients Ingredient Name Strength SODIUM CHLORIDE (UNII: 451W47IQ8X) SODIUM PHOSPHATE, DIBASIC (UNII: GR686LBA74) SODIUM PHOSPHATE, MONOBASIC, MONOHYDRATE (UNII: 593YOG76RN) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:0498-0100-02 118 mL in 1 BOTTLE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M018 12/18/2018 Part 2 of 4 ALCOHOL WIPE
isopropyl alcohol swabProduct Information Item Code (Source) NDC:0498-0143 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ISOPROPYL ALCOHOL (UNII: ND2M416302) (ISOPROPYL ALCOHOL - UNII:ND2M416302) ISOPROPYL ALCOHOL 0.7 mL in 1 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:0498-0143-04 0.4 mL in 1 POUCH; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved drug other 09/18/2018 Part 3 of 4 PVP IODINE WIPE
povidone-iodine 10% swabProduct Information Item Code (Source) NDC:0498-0121 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength POVIDONE-IODINE (UNII: 85H0HZU99M) (IODINE - UNII:9679TC07X4) IODINE 10 mg in 1 mL Inactive Ingredients Ingredient Name Strength NONOXYNOL-9 (UNII: 48Q180SH9T) WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:0498-0121-00 0.3 mL in 1 POUCH; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved drug other 09/18/2018 Part 4 of 4 BURN JEL
lidocaine hydrochloride gelProduct Information Item Code (Source) NDC:59898-200 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength LIDOCAINE HYDROCHLORIDE (UNII: V13007Z41A) (LIDOCAINE - UNII:98PI200987) LIDOCAINE HYDROCHLORIDE ANHYDROUS 2 g in 100 mL Inactive Ingredients Ingredient Name Strength DIAZOLIDINYL UREA (UNII: H5RIZ3MPW4) EDETATE DISODIUM (UNII: 7FLD91C86K) PROPYLPARABEN (UNII: Z8IX2SC1OH) OCTOXYNOL-9 (UNII: 7JPC6Y25QS) DIPROPYLENE GLYCOL (UNII: E107L85C40) TEA TREE OIL (UNII: VIF565UC2G) GLYCERIN (UNII: PDC6A3C0OX) METHYLPARABEN (UNII: A2I8C7HI9T) TROLAMINE (UNII: 9O3K93S3TK) CARBOMER HOMOPOLYMER TYPE C (ALLYL PENTAERYTHRITOL CROSSLINKED) (UNII: 4Q93RCW27E) CARBOMER HOMOPOLYMER TYPE B (ALLYL PENTAERYTHRITOL CROSSLINKED) (UNII: HHT01ZNK31) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:59898-200-12 118 mL in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved drug other 04/17/2010 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved drug other 10/18/2018 Labeler - Honeywell Safety Products USA, INC (118768815)