Label: CLINIQUE EVEN BETTER CLINICAL SERUM FOUNDATION BROAD SPECTRUM SPF 25- titanium dioxide, zinc oxide liquid
- NDC Code(s): 49527-080-01, 49527-080-02, 49527-080-03
- Packager: CLINIQUE LABORATORIES LLC
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated October 19, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- SPL UNCLASSIFIED SECTION
- Active ingredients
- Purpose
- Use
- Warnings
-
Directions
For sunscreen use:
- apply liberally 15 minutes before sun exposure
- reapply at least every two hours
- use a water resistant sunscreen if swimming or sweating
-
Sun Protection Measures. Spending time in the sun increases your risk of skin cancer and early skin aging. To decrease this risk, regularly use a sunscreen with a Broad Spectrum SPF value of 15 or higher and other sun protection measures including:
- limit time in the sun, especially from 10 a.m. – 2 p.m.
- wear long-sleeved shirts, pants, hats and sunglasses
- children under 6 months of age: ask a doctor
-
Inactive ingredients
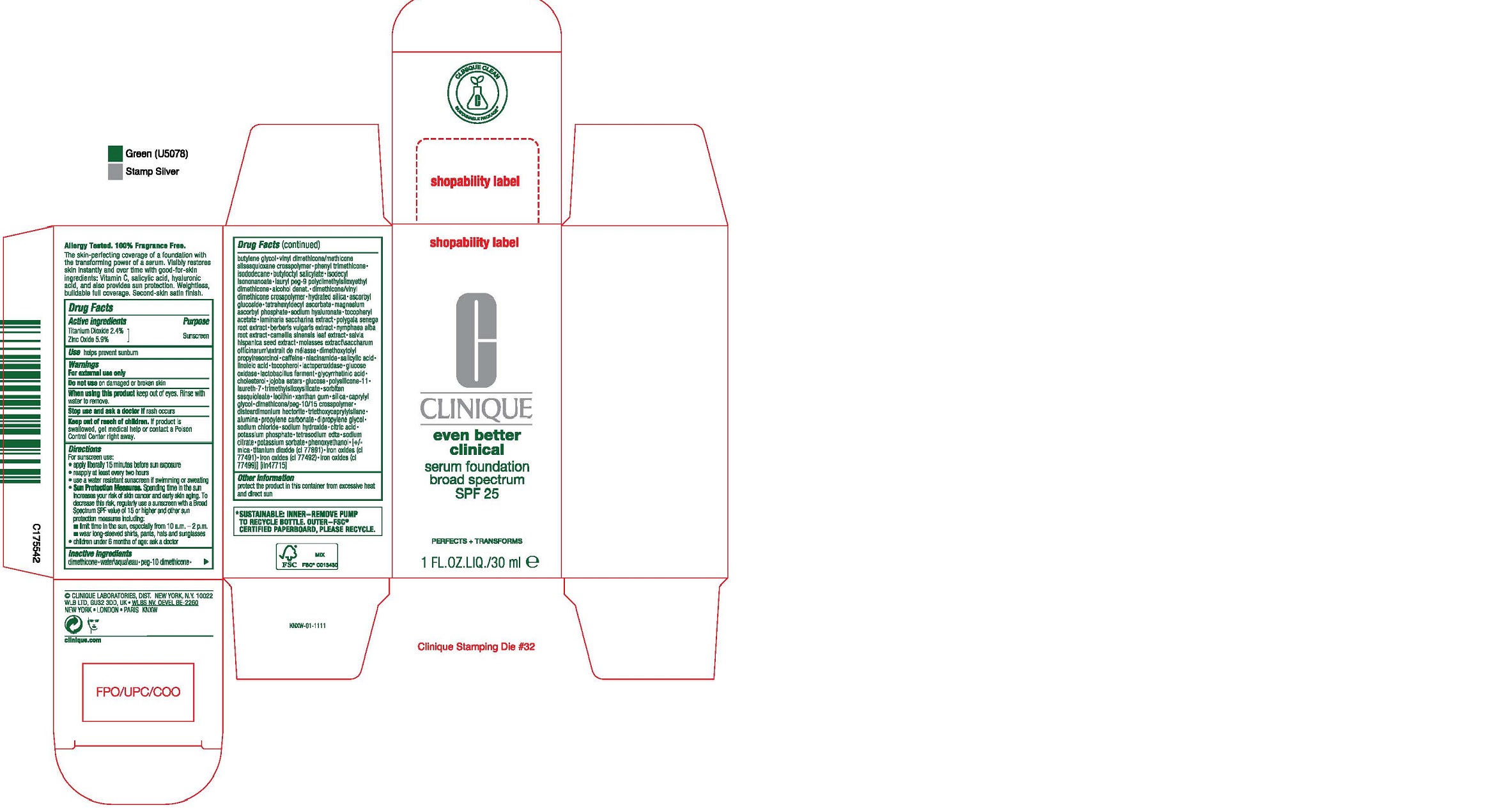
dimethicone•water\aqua\eau•peg-10 dimethicone•butylene glycol•vinyl dimethicone/methicone silsesquioxane crosspolymer•phenyl trimethicone• isododecane•butyloctyl salicylate•isodecyl isononanoate•lauryl peg-9 polydimethylsiloxyethyl dimethicone•alcohol denat.•dimethicone/vinyl dimethicone crosspolymer•hydrated silica•ascorbyl glucoside•tetrahexyldecyl ascorbate•magnesium ascorbyl phosphate•sodium hyaluronate•tocopheryl acetate•laminaria saccharina extract•polygala senega root extract•berberis vulgaris extract•nymphaea alba root extract•camellia sinensis leaf extract•salvia hispanica seed extract•molasses extract\saccharum officinarum\extrait de mélasse•dimethoxytolyl propylresorcinol•caffeine•niacinamide•salicylic acid• linoleic acid•tocopherol•lactoperoxidase•glucose oxidase•lactobacillus ferment•glycyrrhetinic acid• cholesterol•jojoba esters•glucose•polysilicone-11• laureth-7•trimethylsiloxysilicate•sorbitan sesquioleate•lecithin•xanthan gum•silica•caprylyl glycol•dimethicone/peg-10/15 crosspolymer• disteardimonium hectorite•triethoxycaprylylsilane• alumina•propylene carbonate•dipropylene glycol• sodium chloride•sodium hydroxide•citric acid• potassium phosphate•tetrasodium edta•sodium citrate•potassium sorbate•phenoxyethanol•[+/-mica•titanium dioxide (ci 77891)•iron oxides (ci 77491)•iron oxides (ci 77492)•iron oxides (ci 77499)] [iln47715]
- Other information
- PRINCIPAL DISPLAY PANEL - 30 ml Bottle Carton
-
INGREDIENTS AND APPEARANCE
CLINIQUE EVEN BETTER CLINICAL SERUM FOUNDATION BROAD SPECTRUM SPF 25
titanium dioxide, zinc oxide liquidProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:49527-080 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength TITANIUM DIOXIDE (UNII: 15FIX9V2JP) (TITANIUM DIOXIDE - UNII:15FIX9V2JP) TITANIUM DIOXIDE 24 mg in 1 mL ZINC OXIDE (UNII: SOI2LOH54Z) (ZINC OXIDE - UNII:SOI2LOH54Z) ZINC OXIDE 59 mg in 1 mL Inactive Ingredients Ingredient Name Strength NYMPHAEA ALBA ROOT (UNII: 866B05KWBR) GREEN TEA LEAF (UNII: W2ZU1RY8B0) CHIA SEED (UNII: NU0OLX06F8) MOLASSES (UNII: LSU3YX0KZO) DIMETHOXYTOLYL PROPYLRESORCINOL (UNII: 4OD5GIH1UK) CAFFEINE (UNII: 3G6A5W338E) NIACINAMIDE (UNII: 25X51I8RD4) SALICYLIC ACID (UNII: O414PZ4LPZ) LINOLEIC ACID (UNII: 9KJL21T0QJ) TOCOPHEROL (UNII: R0ZB2556P8) MYELOPEROXIDASE (UNII: JQZ6YM58U5) GLUCOSE OXIDASE (UNII: 0T8392U5N1) LIMOSILACTOBACILLUS REUTERI (UNII: 9913I24QEE) ENOXOLONE (UNII: P540XA09DR) CHOLESTEROL (UNII: 97C5T2UQ7J) HYDROGENATED JOJOBA OIL, RANDOMIZED (UNII: Q47ST02F58) ANHYDROUS DEXTROSE (UNII: 5SL0G7R0OK) DIMETHICONE/VINYL DIMETHICONE CROSSPOLYMER (SOFT PARTICLE) (UNII: 9E4CO0W6C5) LAURETH-7 (UNII: Z95S6G8201) TRIMETHYLSILOXYSILICATE (M/Q 0.6-0.8) (UNII: 5041RX63GN) SORBITAN SESQUIOLEATE (UNII: 0W8RRI5W5A) EGG PHOSPHOLIPIDS (UNII: 1Z74184RGV) XANTHAN GUM (UNII: TTV12P4NEE) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) CAPRYLYL GLYCOL (UNII: 00YIU5438U) DIMETHICONE PEG-15 ACETATE (UNII: 11034H9BGQ) DISTEARDIMONIUM HECTORITE (UNII: X687XDK09L) TRIETHOXYCAPRYLYLSILANE (UNII: LDC331P08E) ALUMINUM OXIDE (UNII: LMI26O6933) PROPYLENE CARBONATE (UNII: 8D08K3S51E) DIPROPYLENE GLYCOL (UNII: E107L85C40) SODIUM CHLORIDE (UNII: 451W47IQ8X) SODIUM HYDROXIDE (UNII: 55X04QC32I) CITRIC ACID MONOHYDRATE (UNII: 2968PHW8QP) POTASSIUM PHOSPHATE, UNSPECIFIED FORM (UNII: B7862WZ632) EDETATE SODIUM (UNII: MP1J8420LU) SODIUM CITRATE, UNSPECIFIED FORM (UNII: 1Q73Q2JULR) POTASSIUM SORBATE (UNII: 1VPU26JZZ4) PHENOXYETHANOL (UNII: HIE492ZZ3T) MICA (UNII: V8A1AW0880) FERRIC OXIDE RED (UNII: 1K09F3G675) WATER (UNII: 059QF0KO0R) DIMETHICONE (UNII: 92RU3N3Y1O) DIMETHICONE PEG-10 PHOSPHATE (UNII: O7Q5NJ7X88) BUTYLENE GLYCOL (UNII: 3XUS85K0RA) VINYL DIMETHICONE/METHICONE SILSESQUIOXANE CROSSPOLYMER (UNII: 9NH1UDD2RR) PHENYL TRIMETHICONE (UNII: DR0K5NOJ4R) ISODODECANE (UNII: A8289P68Y2) BUTYLOCTYL SALICYLATE (UNII: 2EH13UN8D3) ISODECYL ISONONANOATE (UNII: 4X46Q4U00Z) LAURYL PEG-9 POLYDIMETHYLSILOXYETHYL DIMETHICONE (UNII: 25G622K2RA) ALCOHOL (UNII: 3K9958V90M) DIMETHICONE/VINYL DIMETHICONE CROSSPOLYMER (HARD PARTICLE) (UNII: H895X08VNQ) HYDRATED SILICA (UNII: Y6O7T4G8P9) ASCORBYL GLUCOSIDE (UNII: 2V52R0NHXW) TETRAHEXYLDECYL ASCORBATE (UNII: 9LBV3F07AZ) MAGNESIUM ASCORBYL PHOSPHATE (UNII: 0R822556M5) HYALURONATE SODIUM (UNII: YSE9PPT4TH) .ALPHA.-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) SACCHARINA LATISSIMA (UNII: 68CMP2MB55) POLYGALA SENEGA ROOT (UNII: M7T6H7D4IF) BERBERIS VULGARIS ROOT BARK (UNII: 1TH8Q20J0U) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:49527-080-01 1 in 1 CARTON 09/24/2020 1 30 mL in 1 BOTTLE; Type 0: Not a Combination Product 2 NDC:49527-080-02 1 in 1 CARTON 06/06/2022 2 5 mL in 1 TUBE; Type 0: Not a Combination Product 3 NDC:49527-080-03 0.2 mL in 1 PACKET; Type 0: Not a Combination Product 11/30/2022 10/19/2023 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M020 09/24/2020 Labeler - CLINIQUE LABORATORIES LLC (044475127) Registrant - Estee Lauder Companies Inc. (790802086) Establishment Name Address ID/FEI Business Operations Estee Lauder Cosmetics Ltd. 202952982 manufacture(49527-080) Establishment Name Address ID/FEI Business Operations Estee Lauder Cosmetics Ltd. 204132062 pack(49527-080) , label(49527-080) , manufacture(49527-080)

 CLINIQUE
CLINIQUE