Label: MONOBASIC SODIUM PHOSPHATE AND DIBASIC SODIUM PHOSPHATE tablet
-
Contains inactivated NDC Code(s)
NDC Code(s): 40032-030-01 - Packager: Novel Laboratories, Inc.
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated January 20, 2012
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Medication Guide: HTML
- Official Label (Printer Friendly)
- SPL UNCLASSIFIED SECTION
-
BOXED WARNING
(What is this?)
BOXED WARNING
There have been rare, but serious reports of acute phosphate nephropathy in patients who received oral sodium phosphate products for colon cleansing prior to colonoscopy. Some cases have resulted in permanent impairment of renal function and some patients required long-term dialysis. While some cases have occurred in patients without identifiable risk factors, patients at increased risk of acute phosphate nephropathy may include those with increased age, hypovolemia, increased bowel transit time (such as bowel obstruction), active colitis, or baseline kidney disease, and those using medicines that affect renal perfusion or function (such as diuretics, angiotensin converting enzyme [ACE] inhibitors, angiotensin receptor blockers [ARBs], and possibly nonsteriodal anti-inflammatory drugs [NSAIDs]). See WARNINGS.
It is important to use the dose and dosing regimen as recommended (pm/am split dose). SeeDOSAGE and ADMINISTRATION.
-
DESCRIPTION
Monobasic Sodium Phosphate and Dibasic Sodium Phosphate Tablet is a purgative used to clean the colon prior to colonoscopy. Monobasic Sodium Phosphate and Dibasic Sodium Phosphate Tablets are manufactured with a highly soluble tablet binder and does not contain microcrystalline cellulose (MCC). Monobasic Sodium Phosphate and Dibasic Sodium Phosphate Tablets are white to off-white modified oval shaped, biconvex, bisect on one side and plain on the other debossed “N” on the left side of bisect and “03” on the right side of the bisect. Each Monobasic Sodium Phosphate and Dibasic Sodium Phosphate Tablet contains 1.102 grams of monobasic sodium phosphate, USP and 0.398 grams of dibasic sodium phosphate, USP for a total of 1.5 grams of sodium phosphate per tablet. Inert ingredients include polyethylene glycol 8000; and magnesium stearate. Monobasic Sodium Phosphate and Dibasic Sodium Phosphate Tablet is gluten-free.
The structural and molecular formulae and molecular weights of the active ingredients are shown below:
Monobasic sodium phosphate, USP
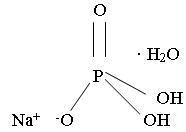
Molecular Formula: NaH2PO4• H2O
Molecular Weight: 137.99
Dibasic sodium phosphate, USP
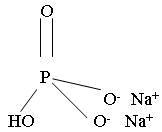
Molecular Formula: Na2HPO4
Molecular Weight: 141.96
Monobasic Sodium Phosphate and Dibasic Sodium Phosphate Tablets are for oral administration only.
-
CLINICAL PHARMACOLOGY
Monobasic sodium phosphate and dibasic sodium phosphate tablets, a dosing regimen containing 48 grams of sodium phosphate (32 tablets), induces diarrhea, which effectively cleanses the entire colon. Each administration has a purgative effect for approximately 1 to 3 hours. The primary mode of action is thought to be through the osmotic effect of sodium, causing large amounts of water to be drawn into the colon, promoting evacuation.
Pharmacokinetics
Pharmacokinetic studies with monobasic sodium phosphate and dibasic sodium phosphate tablets have not been conducted. However, the following pharmacokinetic study was conducted with Visicol tablets which contain the same active ingredients (sodium phosphate) as Monobasic Sodium Phosphate and Dibasic Sodium Phosphate Tablets. In addition, Visicol is administered at a dose that is 25% greater than the monobasic sodium phosphate and dibasic sodium phosphate tablets dose.
An open-label pharmacokinetic study of Visicol in healthy volunteers was performed to determine the concentration-time profile of serum inorganic phosphorus levels after Visicol administration. All subjects received the approved Visicol dosing regimen (60 grams of sodium phosphate with a total liquid volume of 3.6 quarts) for colon cleansing. A 30 gram dose (20 tablets given as 3 tablets every 15 minutes with 8 ounces of clear liquids) was given beginning at 6 PM in the evening. The 30 gram dose (20 tablets given as 3 tablets every 15 minutes with 8 ounces of clear liquids) was repeated the following morning beginning at 6 AM.
Twenty-three healthy subjects (mean age 57 years old; 57% male and 43% female; and 65% Hispanic, 30% Caucasian, and 4% African-American) participated in this pharmacokinetic study. The serum phosphorus level rose from a mean (± standard deviation) baseline of 4.0 (± 0.7) mg/dL to 7.7 (± 1.6 mg/dL), at a median of 3 hours after the administration of the first 30 gram dose of sodium phosphate tablets (see Figure 1). The serum phosphorus level rose to a mean of 8.4 (± 1.9) mg/dL, at a median of 4 hours after the administration of the second 30 gram dose of sodium phosphate tablets. The serum phosphorus level remained above baseline for a median of 24 hours after the administration of the initial dose of sodium phosphate tablets (range 16 to 48 hours).
Figure 1. Mean (± standard deviation) serum phosphorus concentrations
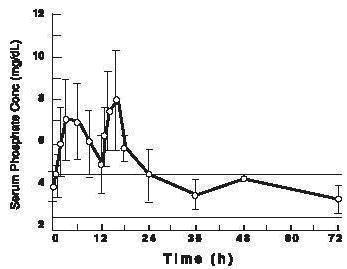
The upper (4.5 mg/dL) and lower (2.6 mg/dL) reference limits for serum phosphate are represented by solid bars.
Special Populations
Renal Insufficiency: The effect of renal dysfunction on the pharmacokinetics of monobasic sodium phosphate and dibasic sodium phosphate tablets has not been studied. Since the inorganic form of phosphate in the circulating plasma is excreted almost entirely by the kidneys, patients with renal disease may have difficulty excreting a large phosphate load. Thus, monobasic sodium phosphate and dibasic sodium phosphate tablets should be used with caution in patients with impaired renal function (see WARNINGS).
Hepatic Insufficiency:
Monobasic sodium phosphate and dibasic sodium phosphate tablets have not been investigated in patients with hepatic failure.
Geriatric:
In a single pharmacokinetic study of sodium phosphate tablets, which included 6 elderly volunteers, plasma half-life increased two-fold in subjects >70 years of age compared to subjects <50 years of age (3 subjects and 5 subjects, respectively).
Gender:
No difference in serum phosphate AUC values were observed in the single pharmacokinetic study conducted with sodium phosphate tablets in 13 male and 10 female healthy volunteers.
-
CLINICAL STUDIES
The colon-cleansing efficacy and safety of monobasic sodium phosphate and dibasic sodium phosphate tablets was evaluated in 2 randomized, investigator-blinded, actively-controlled, multi center, U.S. trials in patients scheduled to have an elective colonoscopy. The trials consisted of a dose ranging and a confirmatory phase 3 study.
In the phase 3 trial, patients were randomized into one of the following three sodium phosphate treatment groups: 1) Visicol containing 60 grams of sodium phosphate given in split doses (30 grams in the evening before the colonoscopy and 30 grams on the next day) with at least 3.6 quarts of clear liquids; 2) monobasic sodium phosphate and dibasic sodium phosphate tablets containing 60 grams of sodium phosphate given in split doses (30 grams in the evening before the colonoscopy and 30 grams on the next day) with 2.5 quarts of clear liquids; and 3) Monobasic sodium phosphate and dibasic sodium phosphate tablets containing 48 grams of sodium phosphate (30 grams in the evening before the colonoscopy and 18 grams on the next day) with 2 quarts of clear liquids. Patients were instructed to eat a light breakfast before noon on the day prior to the colonoscopy and then were told to drink only clear liquids after noon on the day prior to the colonoscopy.
The primary efficacy endpoint was the overall colon cleansing response rate in the 4-point Colonic Contents Scale. Response was defined as a rating of “excellent” or “good” on the 4-point scale as determined by the blinded colonoscopist. This phase 3 study was planned to assess the non-inferiority of the two monobasic sodium phosphate and dibasic sodium phosphate tablets groups compared to the Visicol group.
-
The efficacy analysis included 704 adult patients who had an elective colonoscopy. Patients ranged in age from 21 to 89 years old (mean age 56 years old) with 55% female and 45% male patients. Race was distributed as follows: 87% Caucasian, 10% African American, and 3% other race. The monobasic sodium phosphate and dibasic sodium phosphate tablets 60 gram and 48 gram treatment groups demonstrated non-inferiority compared to Visicol. See Table 1 for the results.
Table 1: Phase 3 study- Overall Colon Content Cleansing Response Rates1 Treatment arm(grams of sodiumphosphate) No. of tablets taken at 6 PM on the day prior to colonoscopy No. of tablets taken the next day2 Excellent Good Fair Inadequate Overall response rate (excellent or good) Monobasic Sodium Phosphate and Dibasic Sodium Phosphate Tablet 32 tabs (48 g) n=236 20 12 76% 19% 3% 2% 95% Monobasic Sodium Phosphate and Dibasic Sodium Phosphate Tablet 40 tabs (60 g) n=233 20 20 73% 24% 2% 1% 97% Visicol40tabs (60 g) n=235 20 20 51% 43% 6% 0% 94% 1Colon-cleansing efficacy was based on response rate to treatment. A patient was considered to be a responder if overall colon cleansing was rated as “excellent” or “good” on a 4-point scale based on the amount of retained “colonic contents”. Excellent was defined as >90% of mucosa seen, mostly liquid stool, minimal suctioning need for adequate visualization. Good was defined as >90% of mucosa seen, mostly liquid stool, significant suctioning needed for adequate visualization. Fair was defined as >90% of mucosa seen, mixture of liquid and semisolid stool, could be suctioned and/or washed. Inadequate was defined as <90% of mucosa seen, mixture of semisolid and/or solid stool which could not be suctioned or washed.
2On the day of the colonoscopy, study medication was taken 3 to 5 hours before the start of the colonoscopy.
Electrolyte Changes
In the monobasic sodium phosphate and dibasic sodium phosphate tablets clinical studies, expected serum electrolyte changes (including phosphate, calcium, potassium, and sodium levels) have been observed in patients taking monobasic sodium phosphate and dibasic sodium phosphate tablets. In the overwhelming majority of patients, electrolyte abnormalities were not associated with any adverse events.
In the monobasic sodium phosphate and dibasic sodium phosphate tablets phase 3 study, 96%, 96%, and 93% of patients who took 60 grams of Visicol, 60 grams of monobasic sodium phosphate and dibasic sodium phosphate tablets, and 48 grams of monobasic sodium phosphate and dibasic sodium phosphate tablets, respectively, developed hyperphosphatemia (defined as phosphate level > 5.1 mg/dL) on the day of the colonoscopy. In this study, patients who took 60 grams of Visicol, 60 grams of monobasic sodium phosphate and dibasic sodium phosphate tablets, and 48 grams of monobasic sodium phosphate and dibasic sodium phosphate tablets had baseline mean phosphate levels of 3.5, 3.5, and 3.6 mg/dL and subsequently developed mean phosphate levels of 7.6, 7.9, and 7.1 mg/dL, respectively, on the day of the colonoscopy.
In the monobasic sodium phosphate and dibasic sodium phosphate tablets phase 3 study, 20%, 22%, and 18% of patients who took 60 grams of Visicol, 60 grams of monobasic sodium phosphate and dibasic sodium phosphate tablets, and 48 grams of monobasic sodium phosphate and dibasic sodium phosphate tablets, respectively, developed hypokalemia (defined as a potassium level <3.4 mEq/L) on the day of the colonoscopy. In this study, patients who took 60 grams of Visicol, 60 grams of monobasic sodium phosphate and dibasic sodium phosphate tablets, and 48 grams of monobasic sodium phosphate and dibasic sodium phosphate tablets all had baseline potassium levels of about 4.3 mEq/L and then developed a mean potassium level of 3.7 mEq/L on the day of the colonoscopy.
In the monobasic sodium phosphate and dibasic sodium phosphate tablets phase 3 trial, several patients on all three sodium phosphate regimens developed hypocalcemia and hypernatremia that did not require treatment.
-
- INDICATIONS AND USAGE
-
CONTRAINDICATIONS
Monobasic sodium phosphate and dibasic sodium phosphate tablets are contraindicated in patients with biopsy-proven acute phosphate nephropathy.
Monobasic sodium phosphate and dibasic sodium phosphate tablets are contraindicated in patients with a known allergy or hypersensitivity to sodium phosphate salts or any of its ingredients.
-
WARNINGS
Administration of sodium phosphate products prior to colonoscopy for colon cleansing has resulted in fatalities due to significant fluid shifts, severe electrolyte abnormalities, and cardiac arrhythmias. These fatalities have been observed in patients with renal insufficiency, in patients with bowel perforation, and in patients who misused or overdosed sodium phosphate products. It is recommended that patients receiving monobasic sodium phosphate and dibasic sodium phosphate tablets be advised to adequately hydrate before, during, and after the use of monobasic sodium phosphate and dibasic sodium phosphate tablets.
Considerable caution should be advised before monobasic sodium phosphate and dibasic sodium phosphate tablets are used in patients with the following illnesses: severe renal insufficiency (creatinine clearance less than 30 mL/minute), congestive heart failure, ascites, unstable angina, gastric retention, ileus, acute bowel obstruction, pseudo-obstruction of the bowel, severe chronic constipation, bowel perforation, acute colitis, toxic megacolon, gastric bypass or stapling surgery, or hypomotility syndrome.
Consider performing baseline and post-colonoscopy labs (phosphate, calcium, potassium, sodium, creatinine, and BUN) in patients who may be at increased risk for serious adverse events, including those with history of renal insufficiency, history of-or at greater risk of-acute phosphate nephropathy, known or suspected electrolyte disorders, seizures, arrhythmias, cardiomyopathy, prolonged QT, recent history of a MI and those with known or suspected hyperphosphatemia, hypocalcemia, hypokalemia, and hypernatremia. Also if patients develop vomiting and/or signs of dehydration then measure post-colonoscopy labs (phosphate, calcium, potassium, sodium, creatinine, and BUN).
Renal Disease, Acute Phosphate Nephropathy, and Electrolyte Disorders
There have been rare, but serious, reports of renal failure, acute phosphate nephropathy, and nephrocalcinosis in patients who received oral sodium phosphate products (including oral sodium phosphate solutions and tablets) for colon cleansing prior to colonoscopy. These cases often resulted in permanent impairment of renal function and several patients required long-term dialysis. The time to onset is typically within days; however, in some cases, the diagnosis of these events has been delayed up to several months after the ingestion of these products. Patients at increased risk of acute phosphate nephropathy may include patients with the following: hypovolemia, baseline kidney disease, increased age, and patients using medicines that affect renal perfusion or function [such as diuretics, angiotensin converting enzyme (ACE) inhibitors, angiotensin receptor blockers, and possibly nonsteroidal anti-inflammatory drugs (NSAIDs).
Use monobasic sodium phosphate and dibasic sodium phosphate tablets with caution in patients with impaired renal function, patients with a history of acute phosphate nephropathy, known or suspected electrolyte disturbances (such as dehydration), or people taking concomitant medications that may affect electrolyte levels (such as diuretics). Patients with electrolyte abnormalities such as hypernatremia, hyperphosphatemia, hypokalemia, or hypocalcemia should have their electrolytes corrected before treatment with monobasic sodium phosphate and dibasic sodium phosphate tablets.
Seizures
There have been rare reports of generalized tonic-clonic seizures and/or loss of consciousness associated with use of sodium phosphate products in patients with no prior history of seizures. The seizure cases were associated with electrolyte abnormalities (e.g., hyponatremia, hypokalemia, hypocalcemia, and hypomagnesemia) and low serum osmolality. The neurologic abnormalities resolved with correction of fluid and electrolyte abnormalities. Monobasic sodium phosphate and dibasic sodium phosphate tablets should be used with caution in patients with a history of seizures and in patients at higher risk of seizure [patients using concomitant medications that lower the seizure threshold (such as tricyclic antidepressants), patients withdrawing from alcohol or benzodiazepines, or patients with known or suspected hyponatremia].
Cardiac Arrhythmias
There have been rare, but serious, reports of arrhythmias associated with the use of sodium phosphate products. Monobasic sodium phosphate and dibasic sodium phosphate tablets should be used with caution in patients with higher risk of arrhythmias (patients with a history of cardiomyopathy, patients with prolonged QT, patients with a history of uncontrolled arrhythmias, and patients with a recent history of a myocardial infarction). Pre-dose and post-colonoscopy ECGs should be considered in patients with high risk of serious, cardiac arrhythmias.
-
PRECAUTIONS
General
Patients should be instructed to drink 8 ounces of clear liquids with each 4-tablet dose of monobasic sodium phosphate and dibasic sodium phosphate tablets. Patients should take a total of 2 quarts of clear liquids with monobasic sodium phosphate and dibasic sodium phosphate tablets. Inadequate fluid intake, as with any effective purgative, may lead to excessive fluid loss, hypovolemia, and dehydration. Dehydration from purgation may be exacerbated by inadequate oral fluid intake, vomiting, and/or use of diuretics.
Patients should be instructed not to administer additional laxative or purgative agents, particularly additional sodium phosphate-based purgative or enema products.
Prolongation of the QT interval has been observed in some patients who were dosed with sodium phosphate colon preparations. QT prolongation with sodium phosphate tablets has been associated with electrolyte imbalances, such as hypokalemia and hypocalcemia. Monobasic sodium phosphate and dibasic sodium phosphate tablets should be used with caution in patients who are taking medications known to prolong the QT interval, since serious complications may occur. Pre-dose and post-colonoscopy ECGs should be considered in patients with known prolonged QT.
Administration of monobasic sodium phosphate and dibasic sodium phosphate tablets may induce colonic mucosal aphthous ulcerations, since this endoscopic finding was observed with other sodium phosphate cathartic preparations. In the monobasic sodium phosphate and dibasic sodium phosphate tablets clinical program, aphthous ulcers were observed in 3% of patients who took the 48 gram monobasic sodium phosphate and dibasic sodium phosphate tablets dosing regimen. This colonoscopic finding should be considered in patients with known or suspected inflammatory bowel disease.
Because published data suggest that sodium phosphate absorption may be enhanced in patients experiencing an acute exacerbation of chronic inflammatory bowel disease, monobasic sodium phosphate and dibasic sodium phosphate tablets should be used with caution in such patients.
Drug Interactions
Medications administered in close proximity to monobasic sodium phosphate and dibasic sodium phosphate tablets may not be absorbed from the gastrointestinal tract due to the rapid intestinal peristalsis and watery diarrhea induced by the purgative agent.
Carcinogenesis, Mutagenesis, Impairment of Fertility
Long-term studies in animals have not been performed to evaluate the carcinogenic potential of monobasic sodium phosphate and dibasic sodium phosphate tablets. Studies to evaluate the effect of monobasic sodium phosphate and dibasic sodium phosphate tablets on fertility or its mutagenic potential have not been performed.
Pregnancy
Teratogenic Effects
Pregnancy Category C
Animal reproduction studies have not been conducted with monobasic sodium phosphate and dibasic sodium phosphate tablets. It is not known whether monobasic sodium phosphate and dibasic sodium phosphate tablets can cause fetal harm when administered to a pregnant woman, or can affect reproduction capacity. Monobasic sodium phosphate and dibasic sodium phosphate tablets should be given to a pregnant woman only if clearly needed.
Pediatric Use
The safety and efficacy of monobasic sodium phosphate and dibasic sodium phosphate tablets have not been demonstrated in patients less than 18 years of age.
Geriatric Use
In controlled colon preparation trials of monobasic sodium phosphate and dibasic sodium phosphate tablets, 228 (24%) of 931 patients were 65 years of age or older. In addition, 49 (5%) of the 931 patients were 75 years of age or older.
Of the 228 geriatric patients in the trials, 134 patients (59%) received at least 48 grams of monobasic sodium phosphate and dibasic sodium phosphate tablets. Of the 49 patients 75 years old or older in the trials, 27 (55%) patients received at least 48 grams of monobasic sodium phosphate and dibasic sodium phosphate tablets. No overall differences in safety or effectiveness were observed between geriatric patients and younger patients. However, the mean phosphate levels in geriatric patients were greater than the phosphate levels in younger patients after monobasic sodium phosphate and dibasic sodium phosphate tablets administration. The mean colonoscopy-day phosphate levels in patients 18-64, 65-74, and ≥75 years old who received 48 grams of monobasic sodium phosphate and dibasic sodium phosphate tablets in the phase 3 study were 7.0, 7.3, and 8.0 mg/dL, respectively. In addition, in all three sodium phosphate treatment groups, the mean phosphate levels in patients 18-64, 65-74, and ≥ 75 years old in the phase 3 study were 7.4, 7.9, and 8.0 mg/dL, respectively, after sodium phosphate administration. Greater sensitivity of some older individuals cannot be ruled out; therefore, monobasic sodium phosphate and dibasic sodium phosphate tablets should be used with caution in geriatric patients.
Sodium phosphate is known to be substantially excreted by the kidney, and the risk of adverse reactions with sodium phosphate may be greater in patients with impaired renal function. Since geriatric patients are more likely to have impaired renal function, consider performing baseline and post-colonoscopy labs (phosphate, calcium, potassium, sodium, creatinine, and BUN) in these patients (see WARNINGS). It is recommended that patients receiving monobasic sodium phosphate and dibasic sodium phosphate tablets be advised to adequately hydrate before, during, and after the use of monobasic sodium phosphate and dibasic sodium phosphate tablets.
-
ADVERSE REACTIONS
Abdominal bloating, abdominal pain, nausea, and vomiting were the most common adverse events reported with the use of monobasic sodium phosphate and dibasic sodium phosphate tablets. Dizziness and headache were reported less frequently. Since diarrhea was considered as a part of the efficacy of monobasic sodium phosphate and dibasic sodium phosphate tablets, diarrhea was not defined as an adverse event in the clinical studies. Table 2 shows the most common adverse events associated with the use of 48 grams of monobasic sodium phosphate and dibasic sodium phosphate tablets, 60 grams of monobasic sodium phosphate and dibasic sodium phosphate tablets, and 60 grams of Visicol in the colon preparation trials (n=931).
Table 2: Frequency of Adverse Events of Any Severity Occurring in Greater than 3% of Patients in the Monobasic Sodium Phosphate and Dibasic Sodium Phosphate TabletsTrials Monobasic Sodium Phosphate and Dibasic Sodium Phosphate Tablets 32 tabs (48 g)N=272 Monobasic Sodium Phosphate and Dibasic Sodium Phosphate Tablets 40 tabs (60 g)N=265 Visicol40 tabs (60 g)N=268 Bloating 31% 39% 41% Nausea 26% 37% 30% Abdominal pan 23% 24% 25% Vomiting 4% 10% 9% Postmarketing Experience
In addition to adverse events reported from clinical trials, the following adverse events have been identified during post-approval use of monobasic sodium phosphate and dibasic sodium phosphate tablets. Because they are reported voluntarily from a population of unknown size, estimates of frequency cannot be made. These events have been chosen for inclusion due to either their seriousness, frequency of reporting or causal connection to monobasic sodium phosphate and dibasic sodium phosphate tablets, or a combination of these factors.
General:
Hypersensitivity reactions including anaphylaxis, rash, pruritus, urticaria, throat tightness, bronchospasm, dyspnea, pharyngeal edema, dysphagia, paresthesia and swelling of the lips and tongue, and facial swelling.
Cardiovascular: Arrhythmias
Nervous System: Seizures
Renal:
Renal impairment, increased blood urea nitrogen (BUN), increased creatinine, acute renal failure, acute phosphate nephropathy, nephrocalcinosis, and renal tubular necrosis.
- DRUG ABUSE AND DEPENDENCE
-
OVERDOSAGE
There have been no reported cases of overdosage with monobasic sodium phosphate and dibasic sodium phosphate tablets. Purposeful or accidental ingestion of more than the recommended dosage of monobasic sodium phosphate and dibasic sodium phosphate tablets might be expected to lead to severe electrolyte disturbances, including hyperphosphatemia, hypocalcemia, hypernatremia, or hypokalemia, as well as dehydration and hypovolemia, with attendant signs and symptoms of these disturbances. Certain severe electrolyte disturbances resulting from overdose may lead to cardiac arrhythmias, seizure, renal failure, and death. The patient who has taken an overdosage should be monitored carefully, and treated symptomatically for complications until stable.
-
DOSAGE AND ADMINISTRATION
The recommended dose of monobasic sodium phosphate and dibasic sodium phosphate tablets for colon cleansing for adult patients is 32 tablets (48 grams of sodium phosphate) taken orally with a total of 2 quarts of clear liquids in the following manner:
-
The evening before the colonoscopy procedure:
-
-
Take 4 monobasic sodium phosphate and dibasic sodium phosphate tablets with 8 ounces of clear liquids every 15 minutes for a total of 20 tablets.
-
-
-
On the day of the colonoscopy procedure:
-
-
-
Starting 3-5 hours before the procedure, take 4 monobasic sodium phosphate and dibasic sodium phosphate tablets with 8 ounces of clear liquids every 15 minutes for a total of 12 tablets.
-
Patients should be advised of the importance of taking the recommended fluid regimen. It is recommended that patients receiving monobasic sodium phosphate and dibasic sodium phosphate tablets be advised to adequately hydrate before, during, and after the use of monobasic sodium phosphate and dibasic sodium phosphate tablets.
Patients should not use monobasic sodium phosphate and dibasic sodium phosphate tablets for colon cleansing within seven days of previous administration. No additional enema or laxative is required, and patients should be advised NOT to take additional agents, particularly those containing sodium phosphate.
-
-
HOW SUPPLIED
Monobasic Sodium Phosphate and Dibasic Sodium Phosphate Tablets are supplied in child-resistant bottles containing 100 tablets. Each tablet contains 1.102 g monobasic sodium phosphate, USP and 0.398 g dibasic sodium phosphate, USP for a total of 1.5 g of sodium phosphate per tablet. Each bottle contains two silica desiccant packets, which should not be ingested.
NDC 40032-030-24 (100 tablets)
-
Medguide
Monobasic Sodium Phosphate and Dibasic Sodium Phosphate Tablets
Read the Medication Guide that comes with Monobasic Sodium Phosphate and Dibasic Sodium Phosphate Tablets before you take it and each time you take it. This Medication Guide does not take the place of talking with your doctor about your medical condition or your treatment. If you have any questions about Monobasic Sodium Phosphate and Dibasic Sodium Phosphate Tablets, ask your doctor or pharmacist.
What is the most important information I should know about Monobasic Sodium Phosphate and Dibasic Sodium Phosphate Tablets?
Monobasic Sodium Phosphate and Dibasic Sodium Phosphate Tablets can cause serious side effects, including:
Serious kidney problems. Rare, but serious kidney problems can happen in people who take medicines made with sodium phosphate, including Monobasic Sodium Phosphate and Dibasic Sodium Phosphate Tablets, to clean your colon before a colonoscopy. These kidney problems can sometimes lead to kidney failure or the need for dialysis for a long time. These problems often happen within a few days, but sometimes may happen several months after taking Monobasic Sodium Phosphate and Dibasic Sodium Phosphate Tablets.
Conditions that can make you more at risk for having serious kidney problems with Monobasic Sodium Phosphate and Dibasic Sodium Phosphate Tablets include if you:
-
• lose too much body fluid (dehydration)
-
-
• have slow moving bowels
-
-
-
• have bowels blocked with stool (constipation)
-
-
-
• have severe stomach pain or bloating
-
-
-
• have any disease that causes bowel irritation (colitis)
-
-
-
• have kidney disease
-
-
-
• have heart failure
-
-
-
• take water pills or non-steroidal anti-inflammatory drugs (NSAIDS).
-
Your age may also affect your risk for having kidney problems with Monobasic Sodium Phosphate and Dibasic Sodium Phosphate Tablets.
Before you start taking Monobasic Sodium Phosphate and Dibasic Sodium Phosphate Tablets, tell your doctor if you:
-
• have kidney problems
-
-
• take any medicines for blood pressure, heart disease, or kidney disease.
-
Severe fluid loss. People who take medicines that contain sodium phosphate can have severe loss of body fluid, with severe changes in body salts in the blood, and abnormal heart rhythms. These problems can lead to death.
Tell your doctor if you have any of these symptoms of loss of too much body fluid (dehydration) while taking Monobasic Sodium Phosphate and Dibasic Sodium Phosphate Tablets:
-
• vomiting
-
-
• dizziness
-
-
-
• urinating less often than normal
-
-
-
• headache
-
See "What are the possible side effects of Monobasic Sodium Phosphate and Dibasic Sodium Phosphate Tablets?" for more information about side effects.
What is Monobasic Sodium Phosphate and Dibasic Sodium Phosphate Tablets?
Monobasic Sodium Phosphate and Dibasic Sodium Phosphate Tablets is a prescription medicine used in adults 18 years and older, to clean your colon before a colonoscopy. Monobasic Sodium Phosphate and Dibasic Sodium Phosphate Tablets cleans your colon by causing you to have diarrhea. Cleaning your colon helps your doctor see the inside of your colon more clearly during the colonoscopy.
It is not known if Monobasic Sodium Phosphate and Dibasic Sodium Phosphate Tablets is safe and works in children under age 18.
Who should not take Monobasic Sodium Phosphate and Dibasic Sodium Phosphate Tablets?
Do not take Monobasic Sodium Phosphate and Dibasic Sodium Phosphate Tablets if:
-
• you have had a kidney biopsy that shows you have kidney problems because of too much phosphate
• you are allergic to sodium phosphate salts or any of the ingredients in Monobasic Sodium Phosphate and Dibasic Sodium Phosphate Tablets.
See the end of this Medication Guide for a list of ingredients in Monobasic Sodium Phosphate and Dibasic Sodium Phosphate Tablets.
What should I tell my doctor before taking Monobasic Sodium Phosphate and Dibasic Sodium Phosphate Tablets?
Before taking Monobasic Sodium Phosphate and Dibasic Sodium Phosphate Tablets, tell your doctor about all your medical conditions, including if you have:
-
• any of the medical conditions listed in the section "What is the most important information I should know about Monobasic Sodium Phosphate and Dibasic Sodium Phosphate Tablets?"
-
-
• irritation of the bowel (colitis). Monobasic Sodium Phosphate and Dibasic Sodium Phosphate Tablets can cause symptoms of irritable bowel disease to flare-up.
-
-
-
• damage to your bowels
-
-
-
• problems with abnormal heart beat
-
-
-
• had a recent heart attack or have other heart problems
-
-
-
• symptoms of too much body fluid loss (dehydration) including vomiting, dizziness, urinating less often than normal, or headache
-
-
-
• had stomach surgery
-
-
-
• a history of seizures
-
-
-
• if you drink alcohol
-
-
-
• are on a low salt diet
-
-
-
• are pregnant. It is not known if Monobasic Sodium Phosphate and Dibasic Sodium Phosphate Tablets will harm your unborn baby.
-
Tell your doctor about all the medicines you take, including prescription and non prescription medicines, vitamins, and herbal supplements. Any medicine that you take close to the time that you take Monobasic Sodium Phosphate and Dibasic Sodium Phosphate Tablets may not work as well. Especially tell your doctor if you take:
-
• water pills (diuretics)
-
-
• medicines for blood pressure or heart problems.
-
-
-
• medicines for kidney damage
-
-
-
• medicines for pain, such as aspirin or a non-steroidal anti-inflammatory drug (NSAID)
-
-
-
• a medicine for seizures
-
-
-
• a laxative for constipation in the last 7 days. You should not take another medicine that contains sodium phosphate while you take Monobasic Sodium Phosphate and Dibasic Sodium Phosphate Tablets.
-
Ask your doctor if you are not sure if your medicine is listed above.
Know the medicines you take. Keep a list of your medicines to show your doctor or pharmacist when you get a new prescription.
How should I take Monobasic Sodium Phosphate and Dibasic Sodium Phosphate Tablets?
-
• Take Monobasic Sodium Phosphate and Dibasic Sodium Phosphate Tablets exactly as prescribed by your doctor.
-
-
• It is important for you to drink clear liquids before, during, and after taking Monobasic Sodium Phosphate and Dibasic Sodium Phosphate Tablets. This may help prevent kidney damage. Examples of clear liquids are water, flavored water, lemonade (no pulp), ginger ale, or apple juice. Do not drink any liquids colored purple or red.
-
You must read, understand, and follow these instructions to take Monobasic Sodium Phosphate and Dibasic Sodium Phosphate Tablets the right way:
On the evening before your colonoscopy, you will take a total of 20 Monobasic Sodium Phosphate and Dibasic Sodium Phosphate Tablets, as follows:
- Take 4 Monobasic Sodium Phosphate and Dibasic Sodium Phosphate Tablets with 8 ounces of clear liquids.
- Wait 15 minutes.
- Take 4 more Monobasic Sodium Phosphate and Dibasic Sodium Phosphate Tablets with 8 ounces of clear liquids.
- Repeat steps 2 and 3 above, three more times. Make sure you wait 15 minutes after each time.
On the day of your colonoscopy you will take a total of 12 Monobasic Sodium Phosphate and Dibasic Sodium Phosphate Tablets, starting about 3 to 5 hours before your colonoscopy, as follows:
- Take 4 Monobasic Sodium Phosphate and Dibasic Sodium Phosphate Tablets with 8 ounces of clear liquids.
- Wait 15 minutes.
- Take 4 more Monobasic Sodium Phosphate and Dibasic Sodium Phosphate Tablets with 8 ounces of clear liquids.
- Repeat steps 2 and 3 one more time.
Tell your doctor if you have any of these symptoms while taking Monobasic Sodium Phosphate and Dibasic Sodium Phosphate Tablets:
- vomiting, dizziness, or if you urinate less often than normal. These may be signs that you have lost too much fluid while taking Monobasic Sodium Phosphate and Dibasic Sodium Phosphate Tablets.
- trouble drinking clear fluids
- severe stomach cramping, bloating, nausea, or headache
If you take too much Monobasic Sodium Phosphate and Dibasic Sodium Phosphate Tablets, call your doctor or get medical help right away.
What should I avoid while taking Monobasic Sodium Phosphate and Dibasic Sodium Phosphate Tablets?
- You should not take other laxatives or enemas made with sodium phosphate, while taking Monobasic Sodium Phosphate and Dibasic Sodium Phosphate Tablets.
- You should not use Monobasic Sodium Phosphate and Dibasic Sodium Phosphate Tablets if you have already used it in the last 7 days.
What are the possible side effects of Monobasic Sodium Phosphate and Dibasic Sodium Phosphate Tablets?
Monobasic Sodium Phosphate and Dibasic Sodium Phosphate Tablets can cause serious side effects, including:
- See "What is the most important information I should know about Monobasic Sodium Phosphate and Dibasic Sodium Phosphate Tablets?"
- Seizures or fainting (black-outs). People who take a medicine that contains sodium phosphate, such as Monobasic Sodium Phosphate and Dibasic Sodium Phosphate Tablets, can have seizures or faint (become unconscious) even if they have not had seizures before. Tell your doctor right away if you have a seizure or faint while taking Monobasic Sodium Phosphate and Dibasic Sodium Phosphate Tablets.
- abnormal heart beat (arrhythmias)
- changes in your blood levels of calcium, phosphate, potassium, sodium
The most common side effects of Monobasic Sodium Phosphate and Dibasic Sodium Phosphate Tablets are:
- bloating
- stomach area (abdominal) pain
- nausea
- vomiting
These are not all the possible side effects of Monobasic Sodium Phosphate and Dibasic Sodium Phosphate Tablets. For more information, ask your doctor or pharmacist.
Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088.
How do I store Monobasic Sodium Phosphate and Dibasic Sodium Phosphate Tablets?
- Store Monobasic Sodium Phosphate and Dibasic Sodium Phosphate Tablets at room temperature, between 59° F to 86° F(15° C to 30° C).
- Throw away any Monobasic Sodium Phosphate and Dibasic Sodium Phosphate Tablets that is not needed.
- Keep Monobasic Sodium Phosphate and Dibasic Sodium Phosphate Tablets and all medicines out of the reach of children.
General information about Monobasic Sodium Phosphate and Dibasic Sodium Phosphate Tablets
Medicines are sometimes prescribed for purposes other than those listed in a Medication Guide. Do not use Monobasic Sodium Phosphate and Dibasic Sodium Phosphate Tablets for a condition for which it was not prescribed. Do not give Monobasic Sodium Phosphate and Dibasic Sodium Phosphate Tablets to other people, even if they have the same symptoms that you have. It may harm them.
This Medication Guide summarizes the most important information about Monobasic Sodium Phosphate and Dibasic Sodium Phosphate Tablets. If you would like more information about Monobasic Sodium Phosphate and Dibasic Sodium Phosphate Tablets, talk with your doctor or pharmacist. You can ask your doctor or pharmacist for information that is written for healthcare professionals. For more information, call 1-908-603-6000 or go to www.fda.gov.
What are the ingredients in Monobasic Sodium Phosphate and Dibasic Sodium Phosphate Tablets?
Active ingredients: sodium phosphate monobasic and sodium phosphate dibasic anhydrous
Inactive ingredients: polyethylene glycol 8000 and magnesium stearate
Novel Laboratories, Inc.
Somerset, NJ 08873, USA
NIN-030-00
Rev: 05/2011
-
- PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
MONOBASIC SODIUM PHOSPHATE AND DIBASIC SODIUM PHOSPHATE
monobasic sodium phosphate and dibasic sodium phosphate tabletProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:40032-030 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength SODIUM PHOSPHATE, MONOBASIC, MONOHYDRATE (UNII: 593YOG76RN) (SODIUM CATION - UNII:LYR4M0NH37) SODIUM PHOSPHATE, MONOBASIC, MONOHYDRATE 1.105 g SODIUM PHOSPHATE, DIBASIC ANHYDROUS (UNII: 22ADO53M6F) (SODIUM CATION - UNII:LYR4M0NH37) SODIUM PHOSPHATE, DIBASIC ANHYDROUS 0.398 g Inactive Ingredients Ingredient Name Strength MAGNESIUM STEARATE (UNII: 70097M6I30) POLYETHYLENE GLYCOL 8000 (UNII: Q662QK8M3B) Product Characteristics Color WHITE (white to off-white) Score 2 pieces Shape OVAL (Modified) Size 18mm Flavor Imprint Code N;03 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:40032-030-01 100 in 1 BOTTLE Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA079247 01/20/2012 Labeler - Novel Laboratories, Inc. (793518643) Registrant - Novel Laboratories, Inc. (793518643) Establishment Name Address ID/FEI Business Operations Novel Laboratories, inc. 793518643 MANUFACTURE


