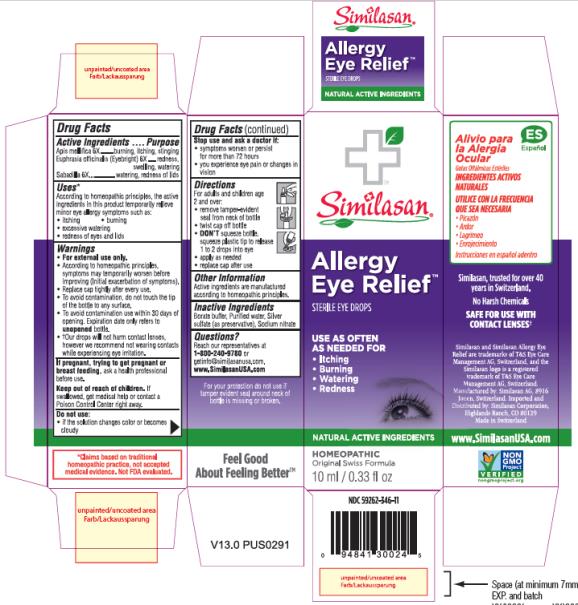
Label: ALLERGY EYE RELIEF- apis mellifera, euphrasia stricta and schoenocaulon officinale seed solution/ drops
- NDC Code(s): 59262-346-11
- Packager: Similasan Corporation
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: unapproved homeopathic
DISCLAIMER: This homeopathic product has not been evaluated by the Food and Drug Administration for safety or efficacy. FDA is not aware of scientific evidence to support homeopathy as effective.
Drug Label Information
Updated October 6, 2021
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- SPL UNCLASSIFIED SECTION
- Active Ingredient
- Purpose
- Active Ingredient
- Purpose
- Active Ingredient
- Purpose
- Uses:
-
Warnings:
-
For external use only.
- According to homeopathic principles, symptoms may temporarily worsen before improving (Initial exacerbation of symptoms).
- Replace cap tightly after every use.
- To avoid contamination, do not touch the plastic tip to any surface.
- To avoid contamination use within 30 days of opening. Expiration date only refers to unopened bottle.
- †Our drops will not harm contact lenses, however we recommend not wearing contacts while experiencing eye irritation.
-
For external use only.
- If pregnant, trying to get pregnant, or breast feeding,
- Keep out of reach of children.
- Do not use:
- Stop use and ask a doctor if:
- Directions:
- Other Information:
- Inactive Ingredients:
- Questions?
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
ALLERGY EYE RELIEF
apis mellifera, euphrasia stricta and schoenocaulon officinale seed solution/ dropsProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:59262-346 Route of Administration OPHTHALMIC Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength APIS MELLIFERA (UNII: 7S82P3R43Z) (APIS MELLIFERA - UNII:7S82P3R43Z) APIS MELLIFERA 6 [hp_X] in 0.45 mL EUPHRASIA STRICTA (UNII: C9642I91WL) (EUPHRASIA STRICTA - UNII:C9642I91WL) EUPHRASIA STRICTA 6 [hp_X] in 0.45 mL SCHOENOCAULON OFFICINALE SEED (UNII: 6NAF1689IO) (SCHOENOCAULON OFFICINALE SEED - UNII:6NAF1689IO) SCHOENOCAULON OFFICINALE SEED 6 [hp_X] in 0.45 mL Inactive Ingredients Ingredient Name Strength BORIC ACID (UNII: R57ZHV85D4) SILVER SULFATE (UNII: 8QG6HV4ZPO) SODIUM NITRATE (UNII: 8M4L3H2ZVZ) WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:59262-346-11 10 mL in 1 BOTTLE, DROPPER; Type 0: Not a Combination Product 01/01/1985 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved homeopathic 01/01/1985 Labeler - Similasan Corporation (111566530)