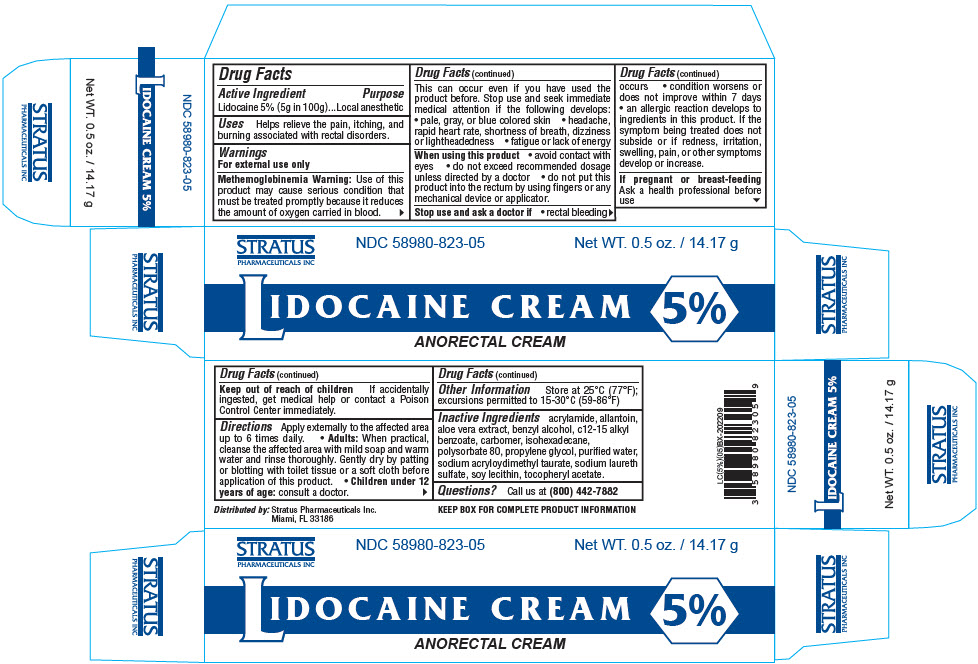
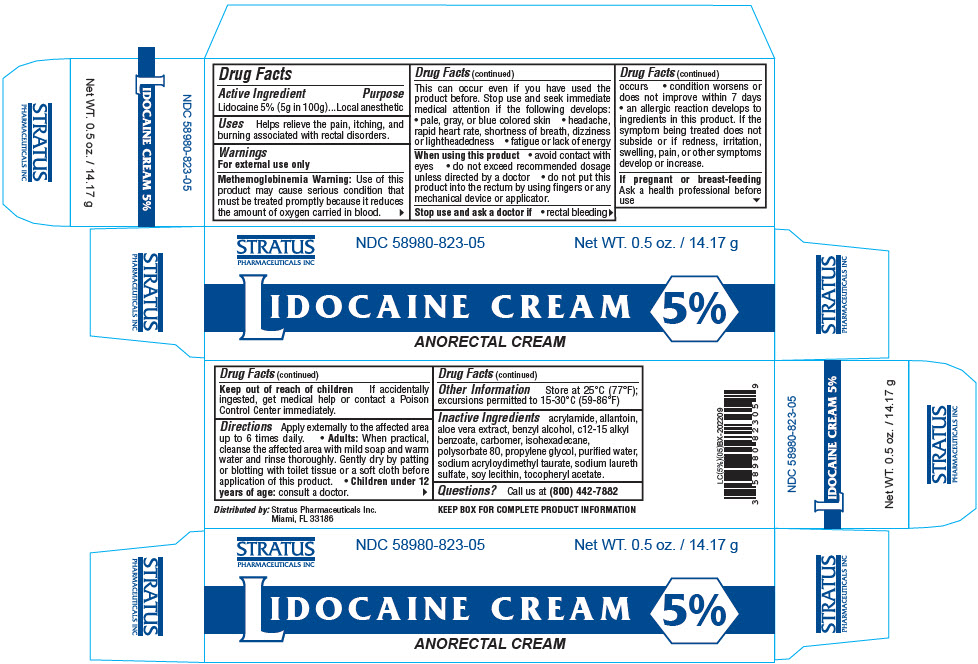
Label: LIDOCAINE cream
- NDC Code(s): 58980-823-05, 58980-823-30
- Packager: STRATUS PHARMACEUTICALS INC
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: OTC monograph final
DISCLAIMER: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
Drug Label Information
Updated September 13, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- SPL UNCLASSIFIED SECTION
- Active Ingredient
- Purpose
- Uses
-
WARNINGS
"Methemoglobinemia Warning" use of this product may cause; a serious condition that must be treated promptly because it reduces the amount of oxygen carried in blood. This can occur even if you have used the product before. Stop use and seek immediate medical attention if the following develops: pale, gray, or blue colored skin (cyanosis), headache, rapid heart rate, shortness of breath, dizziness or lightheadedness, fatigue or lack of energy. If pregnant or breast feeding, ask a health provider before use.
-
Directions
When practical, clean area with mild soap and warm water and rinse thoroughly. Gently dry by patting or blotting with toilet tissue or soft cloth before applying. Adults and children 12 years and older: apply externally to the affected area up to 6 times a day. Children under 12 years of age: consult a doctor. To use finger cots: Roll one finger cot over finger. Gently squeeze cream onto finger cot. Smooth a layer of the cream over affected area.
- Other Information
- Inactive Ingredients
- SPL UNCLASSIFIED SECTION
- PRINCIPAL DISPLAY PANEL - 14.17 g Tube Box
-
INGREDIENTS AND APPEARANCE
LIDOCAINE
lidocaine creamProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:58980-823 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength LIDOCAINE (UNII: 98PI200987) (LIDOCAINE - UNII:98PI200987) LIDOCAINE 50 mg in 1 g Inactive Ingredients Ingredient Name Strength ACRYLAMIDE (UNII: 20R035KLCI) ALLANTOIN (UNII: 344S277G0Z) ALOE VERA LEAF (UNII: ZY81Z83H0X) BENZYL ALCOHOL (UNII: LKG8494WBH) ALKYL (C12-15) BENZOATE (UNII: A9EJ3J61HQ) CARBOMER HOMOPOLYMER, UNSPECIFIED TYPE (UNII: 0A5MM307FC) ISOHEXADECANE (UNII: 918X1OUF1E) POLYSORBATE 80 (UNII: 6OZP39ZG8H) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) WATER (UNII: 059QF0KO0R) SODIUM ACRYLOYLDIMETHYLTAURATE (UNII: 2T9Q6EKI0G) SODIUM LAURETH-3 SULFATE (UNII: BPV390UAP0) LECITHIN, SOYBEAN (UNII: 1DI56QDM62) .ALPHA.-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:58980-823-30 1 in 1 BOX 08/12/2019 1 28.35 g in 1 TUBE; Type 0: Not a Combination Product 2 NDC:58980-823-05 1 in 1 BOX 12/18/2023 2 14.17 g in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC MONOGRAPH FINAL part346 08/12/2019 Labeler - STRATUS PHARMACEUTICALS INC (789001641) Establishment Name Address ID/FEI Business Operations TARMAC PRODUCTS INC 059890491 MANUFACTURE(58980-823)