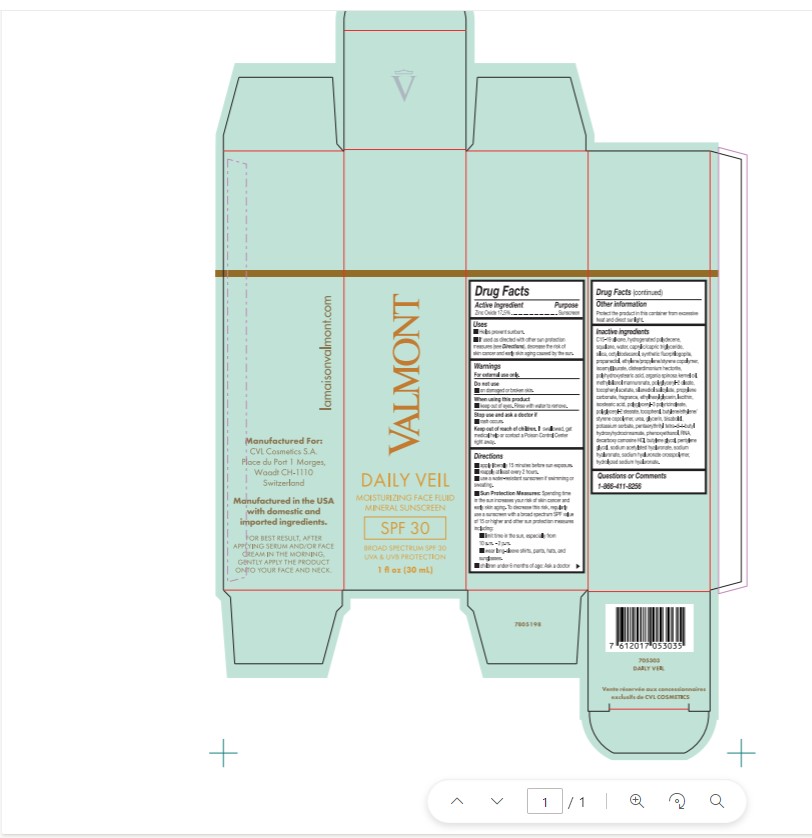
Label: VALMONT DAILY VEIL. MOISTURIZING FACE FLUID MINERAL SUNSCREEN- zinc oxide cream
- NDC Code(s): 73284-508-00
- Packager: Innovation Labs, Inc.
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated October 18, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Active Ingredient
- Purpose
- Uses
- Warnings
- Do not use
- When using this product
- Stop use and ask a doctor if
- Keep out of reach of children.
- Directions
-
Sun Protection Measures:
- Spending time in the sun increases your risk of skin cancer and early skin aging. To decrease this risk, regularly use a sunscreen with broad spectrum SPF value of 15 or higher and other sun protection measures including:
- limit time in the sun, especially from 10 a.m. - 2 p.m.
- wear long-sleeve shirts, pants, hats and sunglasses.
- children under 6 months of age: Ask a doctor
- Other Information
-
Inactive Ingredients
C15-19 alkane, hydrogenated polydecene, squalane, water, caprylic/capric triglyceride, silica, octyldodecanol, synthetic fluorphlogopite, propanediol, ethylene/propylene/styrene copolymer, isoamyl laurate, disteardimonium hectorite, polyhydroxystearic acid, argania spinosa kernel oil, methylsilanol mannuronate, polyglyceryl-2 oleate, tocopheryl acetate, silanediol salicylate, propylene carbonate, fragrance, ethylhexylglycerin, lecithin, isostearic acid, polyglyceryl-3 polyricinoleate, polyglyceryl-2 stearate, tocopherol, butylene/ethylene/styrene copolymer, urea, glycerin, bisabolol, potassium sorbate, pentaerythirtyl tetra-di-t-butyl hydroxyhdrocinnamate, phenoxyethanol, RNA, decarboxy carnosine HCL, butylene glycol, pentylene glycol, sodium acetylated hyaluronate, sodium hyaluronate, sodium hyaluronate crosspolymer, hydrolyzed sodium hyaluronate
- Questions or Comments
- Valmont Daily Veil Moisturizing Face Sunscreen 73248-508-00
-
INGREDIENTS AND APPEARANCE
VALMONT DAILY VEIL. MOISTURIZING FACE FLUID MINERAL SUNSCREEN
zinc oxide creamProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:73284-508 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ZINC OXIDE (UNII: SOI2LOH54Z) (ZINC CATION - UNII:13S1S8SF37) ZINC CATION 175 mg in 1 mL Inactive Ingredients Ingredient Name Strength C15-19 ALKANE (UNII: CI87N1IM01) HYDROGENATED POLYDECENE TYPE I (UNII: U333RI6EB7) SQUALANE (UNII: GW89575KF9) WATER (UNII: 059QF0KO0R) MEDIUM-CHAIN TRIGLYCERIDES (UNII: C9H2L21V7U) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) OCTYLDODECANOL (UNII: 461N1O614Y) MAGNESIUM POTASSIUM ALUMINOSILICATE FLUORIDE (UNII: YK3DC63Y5M) PROPANEDIOL (UNII: 5965N8W85T) ISOAMYL LAURATE (UNII: M1SLX00M3M) DISTEARDIMONIUM HECTORITE (UNII: X687XDK09L) POLYHYDROXYSTEARIC ACID (2300 MW) (UNII: YXH47AOU0F) ARGAN OIL (UNII: 4V59G5UW9X) METHYLSILANOL ASCORBATE (UNII: 46Z5D1I0IS) POLYGLYCERYL-2 OLEATE (UNII: 5759J47SAM) .ALPHA.-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) SILANEDIOL SALICYLATE (UNII: C054DF30K0) PROPYLENE CARBONATE (UNII: 8D08K3S51E) ETHYLHEXYLGLYCERIN (UNII: 147D247K3P) ISOSTEARIC ACID (UNII: X33R8U0062) POLYGLYCERYL-3 PENTARICINOLEATE (UNII: 7Q0OK5DOT4) POLYGLYCERYL-2 STEARATE (UNII: 253MC0P0YV) TOCOPHEROL (UNII: R0ZB2556P8) UREA (UNII: 8W8T17847W) GLYCERIN (UNII: PDC6A3C0OX) LEVOMENOL (UNII: 24WE03BX2T) POTASSIUM SORBATE (UNII: 1VPU26JZZ4) PENTAERYTHRITOL TETRAKIS(3-(3,5-DI-TERT-BUTYL-4-HYDROXYPHENYL)PROPIONATE) (UNII: 255PIF62MS) PHENOXYETHANOL (UNII: HIE492ZZ3T) RNA-BINDING MOTIF PROTEIN 39 (UNII: 7CGZ92983T) DECARBOXY CARNOSINE HYDROCHLORIDE (UNII: 6X7K9I5QR7) BUTYLENE GLYCOL (UNII: 3XUS85K0RA) PENTYLENE GLYCOL (UNII: 50C1307PZG) SODIUM ACETYLATED HYALURONATE (UNII: WN66R7GL93) HYALURONIC ACID (UNII: S270N0TRQY) HYALURONATE SODIUM (UNII: YSE9PPT4TH) SODIUM S-(2-HYDROXY-3-(3-(TRIHYDROXYSILYL)PROPOXY)PROPYL) SULFUROTHIOATE (UNII: 2V56GQW1BM) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:73284-508-00 1 in 1 CARTON 01/07/2024 1 30 mL in 1 BOTTLE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M020 01/07/2024 Labeler - Innovation Labs, Inc. (117109069)